groups in periodic system of elements → skupine periodnog sustava
Periodic system of elements is divided into 18 groups of chemical elements. Elements belonging to the same group have a same number of valence electrons and similar chemical properties. Elements of main groups are in 1., 2., and in groups 13. to 18. Different groups of elements can be named according to the first element in the group (elements of boron group, elements of carbon group), or they have some special names (noble gases, halogenic elements, halyde elements, earthalkali and alkali metals).
haematite → hematit
Haematite is a mineral of iron(III) oxide Fe2O3. It is the most important ore of iron and usually occurs in two main forms: as a massive red kidney-shaped ore and as grey to black metallic crystals known as specular iron ore. Haematite is the major red colouring agent in rocks; the largest deposits are of sedimentary origin. In industry haematite is also used as a polishing agent (jeweller’s rouge) and in paints.
hafnium → hafnij
Hafnium was discovered by Dirk Coster (Denmark) and Georg Karl von Hevesy (Hungary) in 1923. The origin of the name comes from the Latin name Hafnia meaning Copenhagen. It is silvery, ductile metal. Exposed surfaces form oxide film. Resists alkalis and acids (except HF). Toxic. Metal ignites and burns readily. Hafnium is obtained from mineral zircon or baddeleyite. Used in reactor control rods because of its ability to absorb neutrons.
halogens → halogeni elementi
Halogens are the elements fluorine (F) chlorine (Cl), bromine (Br), iodine (I), and astatine (At). They are non-metals, and make up part of the 17 group in the periodic table. Compounds of these elements are called halogenides or halides.
The halogens all have a strong unpleasant odour and will burn flesh. They do not dissolve well in water. The five elements are strongly electronegative. They are oxidising agents, with fluorine being the strongest and astatine being the weakest. They react with most metals and many non-metals.
Halogens form molecules which consist of atoms covalently bonded. With increasing atomic weight there is a gradation in physical properties. For example: Fluorine is a pale green gas of low density. Chlorine is a greenish-yellow gas 1.892 times as dense as fluorine. Bromine is a deep reddish-brown liquid which is three times as dense as water. Iodine is a grayish-black crystalline solid with a metallic appearance. And astatine is a solid with properties which indicate that it is somewhat metallic in character.
thermionic emission → termionska emisija
Thermionic emission is the boiling off of electrons from heated metals. It gives a source of electrons for cathode ray tubes.
thermit welding → termitno zavarivanje
Thermit welding is a group of welding processes in which fusion is produced by heating with superheated liquid metal resulting from a chemical reaction between a metal oxide and aluminium.
toxic chemical → bojni otrov
Toxic chemical means any chemical which through its chemical action on life processes can cause death, temporary incapacitation, or permanent harm to humans or animals. This includes all such chemicals, regardless of their origin or of their method of production, and regardless of whether they are produced in facilities, in munitions or elsewhere.
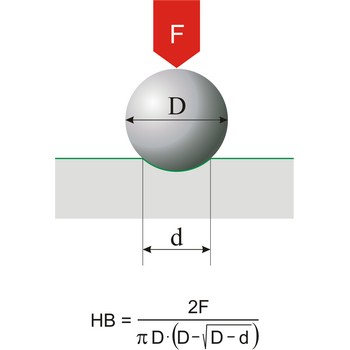
hardness → tvrdoća
Hardness is the resistance of a material to deformation of an indenter of specific size and shape under a known load. This definition applies to all types of hardness scales except Mohs scale, which is a based on the concept of scratch hardness and is used chiefly for minerals. The most generally used hardness scales are Brinell (for cast iron), Rockwell (for sheet metal and heat-treated steel), Knoop (for metals).
hassium → hassij
Hassium was discovered by Peter Armbruster, Gottfried Münzenber and their co-workers at the Heavy Ion Research Laboratory (Gesellschaft für Schwerionenforschung, GSI) in Darmstadt, Germany in 1984. The origin of the name is the Latin word Hassias meaning Hess, the German state. It is synthetic radioactive metal. Hassium was produced bythe bombardment of lead-208 with iron-58.
Citing this page:
Generalic, Eni. "Meta položaj." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table