formaldehyde → formaldehid
Formaldehyde (methanal) is a colourless gas, HCHO; r.d. 0.815 (at -20 °C); m.p. -92 °C; b.p. -21 °C. It is the simplest aldehyde, made by the catalytic oxidation of methanol (500 °C; silver catalyst) by air. It forms two polymers: methanal trimer and polymethanal.
fouling → obrastanje
1. Fouling is the deposition of insoluble materials, such as bacteria, colloids, oxides and water-borne debris, onto the surface of a reverse osmosis or ultrafiltration membrane. Fouling is associated with decreased flux rates and may also reduce the rejection rates of reverse osmosis membranes. Fouling also refers to the accumulation of normally inorganic deposits on heat exchanger tubing.
2. Fouling is an accumulation of marine organism deposits on a submerged metal surface.
calcium → kalcij
Calcium was discovered by Sir Humphry Davy (England) in 1808. The origin of the name comes from the Latin word calix meaning lime. It is fairly hard, silvery-white metal. Exposed surfaces form oxides and nitrides. Reacts with water and oxygen. Occurs only in compounds. Calcium is obtained from minerals like chalk, limestone and marble. Pure metal is produced by replacing the calcium in lime (CaCO3) with aluminium in hot, low pressure retorts. Used by many forms of life to make shells and bones. Virtually no use for the pure metal, however two of its compounds are, lime (CaO) and gypsum (CaSO4), are in great demand by a number of industries.
carat → karat
Carat (ct.) is a metric unit of mass for the weighing of precious metals, precious stone and gems (e.g. diamond, ruby, sapphire).
Carat (ct, Kt) corresponds to the mass fraction of gold in precious alloys used in jewellery.
1 ct = 0.4167 %
24 ct = 100 %
24 carat gold is pure gold, 18 carat is 75 % gold and 25 % of some other metal such as silver or copper.
catalyst → katalizator
Catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. Catalysts that have the same phase as the reactants are homogenous catalysts (e.g. enzymes in biochemical reactions). Those that have a different phase are heterogeneous catalyst (e.g. metals or oxides used in gas reactions).
The catalyst provides an alternative pathway by which the reaction can proceed, in which the activation energy is lower. In thus increases the rate at which the reaction comes to an equilibrium, although it does not alter the position of the equilibrium.
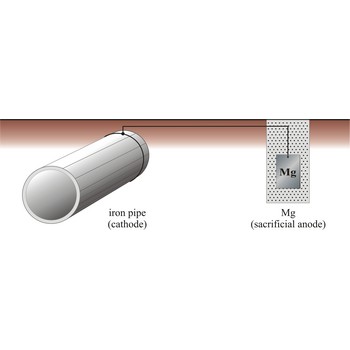
cathodic protection → katodna zaštita
Cathodic protection is a process in which a structural metal, such as iron, is protected from corrosion by connecting it to a metal that has a more negative reduction half-cell potential, which now corrodes instead of iron. There are two major variations of the cathodic method of corrosion protection. The first is called the impressed current method, and the other is called the sacrificial anode method.
fractional crystallisation → frakcijska kristalizacija
Fractional crystallisation is a method of separating a mixture of soluble solids by dissolving them in a suitable hot solvent and then lowering the temperature slowly. The least soluble component will crystallise out first, leaving the other components in the solution. By controlling the temperature, it is sometimes possible to remove each component in turn.
francium → francij
Francium was discovered by Marguerite Perey (France) in 1939. Named for France, the nation of its discovery. It is highly rare and unstable, radioactive metal. Chemical properties similar to cesium. Francium is formed by decay of actinium. Produced by bombarding radium or astatine with neutrons. Since its isotopes have such short half-lives there are no commercially significant compounds of francium.
cerium → cerij
Cerium was discovered by Martin Heinrich Klaproth (Germany) and by Jöns Jacob Berzelius (Sweden) in 1803 and Wilhelm von Hisinger (Germany) in 1814. Named after the asteroid Ceres this discovered two years before the element. It is malleable, ductile, iron-grey metal. Tarnishes in air; reacts easily with water. Dissolves in acids; ignites when heated. Metal ignites and burns readily. Strong reductant. Cerium is most abundant rare earth metal. Found in many minerals like monazite sand [Ce(PO4)]. Its oxides are used in the optics and glass-making industries. Its salts are used in the photography and textile industry. Used in high-intensity carbon lamps and as alloying agents in special metals.
cetane number → cetanski broj
Cetane number is a measure of the ignition quality of diesel fuel. It denotes the volume fraction of cetane (C16H34) in a combustible mixture (containing cetane and 1-methylnapthalene) whose ignition characteristics match those of the diesel fuel being tested. Cetane is a collection of un-branched open chain alkane molecule that ignites very easily under compression, so it was assigned a cetane number of 100, while alpha-methyl naphthalene was assigned a cetane number of 0.
Citing this page:
Generalic, Eni. "Meta položaj." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table