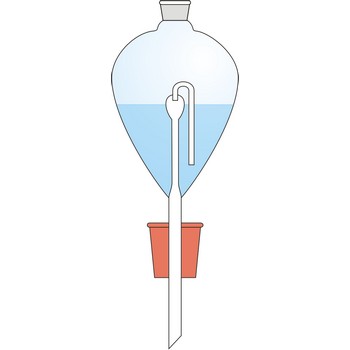
Contat-Gockel’s valve → Contat-Gockelov ventil
Contat-Göckel’s valve is used for maintenance of inert atmosphere in a flask. The valve is filled with a saturated solution of sodium bicarbonate (NaHCO3) so that the end of the tube is covered. Solution inside the valve keeps the flask contents away from the oxygen influence from air. If low pressure is created inside the flask (when the flask is cooled), the solution will penetrate inside it from funnel and in a reaction with acid CO2 is generated which fills up the flask.
Solution from the funnel will keep penetrating until CO2 pressure in the flask is equalised with the outer pressure.
copolymer → kopolimer
Copolymers are also known as heteropolymers. They are made from two (or more) different monomers, which usually undergo a condensation reaction with the elimination of a simple molecule, such as ammonia or water. A typical example is the condensation of 1,6-diaminohexane (hexamethylenediamine) with hexanedioic acid (adipic acid) to form nylon 6,6.
The properties of a polymeric plastic can most easily be modified if it is a copolymer of two or more different monomers, e.g. acrylonitrile-butadiene-styrene copolymer (ABS). Varying the proportions of the component monomers can preselect its properties.
cyclic voltammetry → ciklička voltametrija
Cyclic voltammetry (CV) is an electrochemical measuring technique used for the determination of the kinetics and mechanism of electrode reactions. The potential of the working electrode is controlled (typically with a potentiostat) and the current flowing through the electrode is measured. It is a linear-weep voltammetry with the scan continued in the reverse direction at the end of the first scan. This cycle can be repeated a number of times, and is used for corrosion studies.
Dalton’s atomic theory → Daltonova atomska teorija
Dalton’s atomic theory is a theory of chemical combination, first stated by John Dalton in 1803. It involves the following postulates:
1. Elements consist of indivisible small particles (atoms).
2. All atoms of the same element are identical; different elements have different types of atom.
3. Atoms can neither be created nor destroyed.
4. ’Compound elements’ (i.e. compounds) are formed when atoms of different elements join in simple ratios to form ’compound atoms’ (i.e. molecules).
Dalton also proposed symbols for atoms of different elements (later replaced by the present notation using letters).
intermediate → intermedijer
Intermediate is a molecular or ionic species that is formed (directly or indirectly) from the reactants and reacts further (directly or indirectly) to form the products of the reaction. It does not accumulate during the course of the reaction.
irreversible galvanic cell → nepovrativi galvanski članak
Irreversible galvanic cell is a chemical source of direct current, in which reactions that take place on the electrodes are irreversible.
law of chemical equilibrium → zakon o kemijskoj ravnoteži
Law of chemical equilibrium (also called the law of mass action) states that the rate at which a substance reacts is proportional to its active mass (i.e. to its molar concentration). Thus, the velocity of a chemical reaction is proportional to the product of the concentration of the reactants.
limiting reactant → mjerodavni reaktant
Limiting reactant is a reactant in a chemical reaction that limits the amount of product that can be formed. The reaction will stop when the entire limiting reagent is consumed. These other reactants are present in excess.
differential thermal analysis → diferencijalna termalna analiza
Differential thermal analysis (DTA) is a technique that is often used to analyze materials that react or decompose at higher temperatures. The difference in temperature between the sample and an inert reference material is monitored as both are heated in a furnace. Phase transitions and chemical reactions taking place in the sample on heating cause the temperature difference to become larger, at temperatures that are characteristic of the sample.
Citing this page:
Generalic, Eni. "Lančana reakcija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table