half-wave potential → poluvalni potencijal
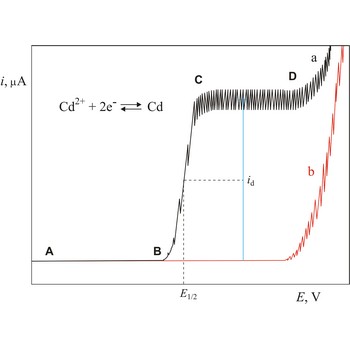
Half-wave potential (E1/2) is a potential at which polarographic wave current is equal to one half of diffusion current (id). In a given supporting electrolyte, the half-wave potential is unique for each element and its different valence states and chemical forms. Observation of a current peak at a specific half-wave potential therefore identifies the chemical species producing the current.
Kirchoff, Gustav → Kirchoff, Gustav
Gustav Kirchoff (1824-1887) was a German physicist who, with the chemist Robert Bunsen (1811-1899), laid the foundations of spectral analysis. He realized that the Fraunhofer lines in the Sun's spectrum were due to light from the photosphere being absorbed at those specific wavelengths by elements in the solar atmosphere. He also found that incandescent solids, liquids, and compressed gases emit a continuous spectrum. Use of the Bunsen burner in conjunction with a glass prism led to the development of the spectroscope in collaboration with the Bunsen and to the spectroscopic discovery of the elements rubidium (1860) and cesium (1861).
polarimetry → polarimetrija
Polarimetry measures the overall turning of the flat of polarised light. It is used when analysing optically active substances and compounds.
spectroscopy → spektroskopija
Spectroscopy is the analysis of the lines of light emitted from excited atoms as the electrons drop back through their orbitals. These lines give the energy and distances of the electronic orbitals.
X-ray crystallography → rendgenska kristalografija
X-ray crystallography is a determination of a three dimensional arrangements of atoms in a crystal by analysis of X-ray diffraction patterns.
Knudsen burette → Knudsenova bireta
Knudsen's automatic bulb-burette, developed by the Danish physicist Martin Knudsen (1871-1949), is designed in a way that even routine field analysis in a boat laboratory would provide highly accurate measurements. The burette is filled with a mixture of silver nitrate from reservoir R, located above the burette, by opening the A valve. When the solution crosses the three-way C valve the A valve is closed preventing further solution flow in to the burette. Any extra solution is caught in the W bowl. Turn the C valve, which marks the zero on the scale, in order to allow atmospheric air to enter the burette. Since most open-ocean samples lie in a relatively small chlorinity range, the burette is designed so that much of its capacity is in the bulb (B). This allows the titration to be quick (by quickly releasing contents from the B area) and reduces the error that occurs from the slow drainage along the inner wall of the burette.
Each millimeter is divided in to twenty parts (double millimeter division of the Knudsen burette) which allows for highly accurate measurements (the scale is read up to a precision of 0.005 mL). From 0 to 16 the burette isn't divided, that usually starts from 16 and goes until 20.5 or 21.5. A single double millimeter on a Knudsen burette scale corresponds to one permille of chloride in the seawater sample. This burette can be used for titration of water from all of the oceans and seas, with the exemptions being areas with very low salinity (e.g. the Baltic Sea) and river estuaries which require the use of normal burettes.
polarogram → polarogram
Polarogram is a graph of current versus potential in a polarographic analysis. The position of a polarographic wave in a polarogram along the x axis (E1/2) provides an identity of the substance while the magnitude of the limiting diffusion current (id) provides the concentration of this substance.
pipette → pipeta
Pipettes are glass tubes which are tapers towards at both ends into narrow opened tubes. According to their design two types of pipettes can be distinguished:
Volumetric pipettes
Volumetric pipettes (transfer or belly pipette) are used in volumetric analysis, when there is a need for taking exact smaller volume of a sample solution or reagent. The upper tube of volumetric pipette has a ringlike marking (mark) which marks its calibrated volume. Pipettes calibrated to deliver (TD or Ex) the indicated volume. By sucking in (with mouth, propipette or a water pump) the liquid is pulled in a little bit above the mark and the opening of the pipet is closed with a forefingertip. Outer wall of pipet is wiped and, with a slight forefinger loosening, the liquid is released until it reaches the mark. Mark must figure as a tangent on a lower edge of the liquid meniscus. A pipette is emptied out by lifting the forefinger off and letting the liquid flow out of the pipette freely. After another 15 s and the tip of the pipette is pulled onto the inner wall of the vessel. It is absolutely forbidden to blow out the contents of the pipette
Graduated pipettes
Graduated pipettes (Mohr pipette) have a scale divided into units of one and of 1/10th of a millilitre. Because of their wide necks it is less accurate than the volumetric pipette. They are used when taking volume of solutions in which accuracy does not have to be very high. They are filled in the same way as volumetric ones and liquid can be gradually released.
polarography → polarografija
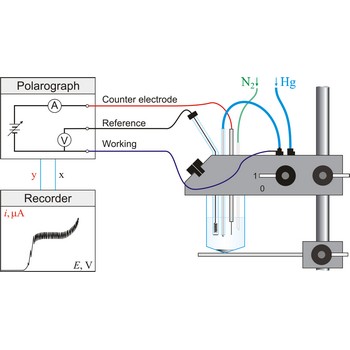
Polarography is a volumetric technique which is based on a diffusion controlled analyte travel to the surface of dropping mercury electrode (DME). The surface of the working electrode (dropping mercury electrode) is constantly renewed under dropping conditions and, thus, the conditions under which reaction takes place are readily reproducible. Depolarisation potential enables identification of ions present in the solution, and by measuring the diffusion current their concentration is calculated. Polarography was discovered in 1922 by the Czech chemist Jaroslav Heyrovský (1890-1967).
Citing this page:
Generalic, Eni. "Kvalitativna analiza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table