Euler number → Eulerova značajka
Euler number (Eu) is a dimensionless quantity used in fluid mechanics, defined by
where p is pressure, ρ is density, and v is velocity.
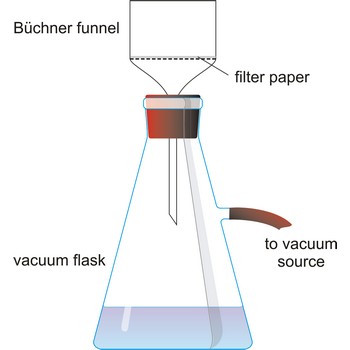
filter flask → boca za odsisavanje
Filter flask, also known as a vacuum flask, is a flask with a side arm to which a vacuum can be applied. It usually have heavy side walls to withstand high vacuum.
Neel temperature → Neelova temperatura
Néel temperature (TN) is the critical temperature above which an antiferromagnetic substance becomes paramagnetic. The phenomenon was discovered around 1930 by the French physicist L.E.F. Néel (1904-2000).
normal boiling point → normalno vrelište
Normal boiling point is a temperature at which pressure of liquid vapour is 101 325 Pa.
osmometry → osmometrija
Osmometry is a determination of the average molecular weight of a dissolved substance from measurements of osmotic pressure.
osmosis → osmoza
Osmosis is the flow of a solvent in a system in which two solutions of different concentration are separated by a semipermeable membrane which cannot pass solute molecules. The solvent will flow from the side of lower concentration to that of higher concentration, thus tending to equalise the concentrations. The pressure that must be applied to the more concentrated side to stop the flow is called the osmotic pressure.
equilibrium constant → konstanta ravnoteže
The equilibrium constant (K) was originally introduced in 1863 by Norwegian chemists C.M. Guldberg and P. Waage using the law of mass action. For a reversible chemical reaction represented by the equation
chemical equilibrium occurs when the rate of the forward reaction equals the rate of the back reaction, so that the concentrations of products and reactants reach steady-state values.
The equilibrium constant is the ratio of chemical activities of the species A, B, C, and D at equilibrium.
To a certain approximation, the activities can be replaced by concentrations.
For gas reactions, partial pressures are used rather than concentrations
The units of Kp and Kc depend on the numbers of molecules appearing in the stoichiometric equation (a, b, c, and d).
The value equilibrium constant depends on the temperature. If the forward reaction is exothermic, the equilibrium constant decreases as the temperature rises. The equilibrium constant shows the position of equilibrium. A low value of K indicates that [C] and [D] are small compared to [A] and [B]; i.e. that the back reaction predominates.
The equilibrium constant is related to ΔrG°, the standard Gibbs free energy change in the reaction, by
polymorphic transition → polimorfni prijelaz
Polymorphic transition is a reversible transition of a solid crystalline phase at a certain temperature and pressure to another phase of the same chemical composition with a different crystal structure. For examples, the transitions of quartz (SiO2) at 1 143 K to tridymite, and at 1 743 K to cristobalite.
Raoult’s law → Raoultov zakon
Raoult’s law is the expression for the vapour pressure pA of component A in an ideal solution, viz.,
where xA is the mole fraction of component A and pAo the vapour pressure of the pure substance A.
Citing this page:
Generalic, Eni. "Kritični tlak." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table