fluid mechanics → mehanika fluida
Fluid mechanics is the study of various properties of the fluid (liquids and gases): velocity, pressure, density and temperature, as functions of space and time.
freezing point → ledište
Freezing point is the temperature at which a liquid becomes a solid at normal atmospheric pressure.
See Melting point
heat of hydration → toplina hidratacije
Heat of hydration or enthalpy of hydration of ions corresponds to the heat that is released by hydration of one mole of ions at a constant pressure. The more the ion is hydrated, the more heat is released. Degree of hydration depends on the size and charge of ion. The smaller the ion and the greater its charge, it will be the more hydrated.
ideal gas → idealni plin
Ideal gas is a gas in which there is complete absence of cohesive forces between the component molecules; the behaviour of such a gas can be predicted accurately by the ideal gas equation through all ranges of temperature and pressure. The concept is theoretical, since no actual gas meets the ideal requirement.
chemical potential → kemijski potencijal
For a mixture of substances, the chemical potential of constituent B (μB) is defined as the partial derivative of the Gibbs energy G with respect to the amount (number of moles) of B, with temperature, pressure, and amounts of all other constituents held constant.
Also called partial molar Gibbs energy. Components are in equilibrium if their chemical potentials are equal.
Clapeyron equation → Clapeyronova jednadžba
Clapeyron equation (also called the Clausius-Clapeyron equation) is a relation between pressure and temperature of two phases of a pure substance that are in equilibrium,
where ΔtrsS is the difference in entropy between the phases and ΔtrsV the corresponding difference in volume.
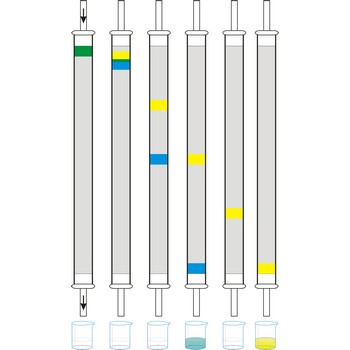
column chromatography → kromatografija u koloni
Column chromatography is generally used as a purification technique: it isolates desired compounds from a mixture. In column chromatography, the stationary phase, a solid adsorbent, is placed in a vertical column. The mobile phase, a liquid, is added to the top and flows down through the column by either gravity or external pressure. The mobile phase can be a gas or a liquid which gives rise to the two basic forms of chromatography, namely, gas chromatography (GC) and liquid chromatography (LC).
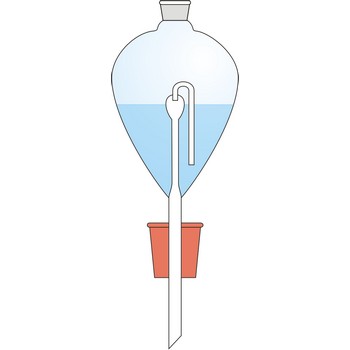
Contat-Gockel’s valve → Contat-Gockelov ventil
Contat-Göckel’s valve is used for maintenance of inert atmosphere in a flask. The valve is filled with a saturated solution of sodium bicarbonate (NaHCO3) so that the end of the tube is covered. Solution inside the valve keeps the flask contents away from the oxygen influence from air. If low pressure is created inside the flask (when the flask is cooled), the solution will penetrate inside it from funnel and in a reaction with acid CO2 is generated which fills up the flask.
Solution from the funnel will keep penetrating until CO2 pressure in the flask is equalised with the outer pressure.
intensive property → intenzivno svojstvo
intensive property is a property that does not change when the amount of sample changes. Examples are density, pressure, temperature, colour.
Citing this page:
Generalic, Eni. "Kritični tlak." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table