reversible process → reverzibilan proces
Reversible process or reaction is those that can be reversed by an infinitesimally small change in conditions. For example, ice and water coexist at 101 325 Pa and 0 °C; a very slight temperature increase causes the ice to melt; a tiny temperature decrease causes the water to freeze. Melting or freezing under these conditions can be considered reversible.
salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
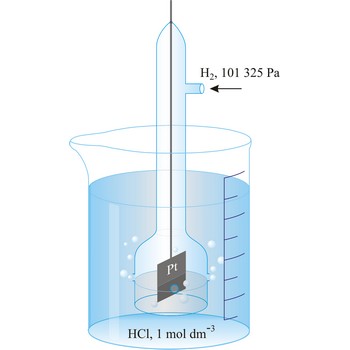
standard electrode potential → standardni elektrodni potencijal
Standard electrode potential (E°) (standard reduction potentials) are defined by measuring the potential relative to a standard hydrogen electrode using 1 mol solution at 25 °C. The convention is to designate the cell so that the oxidised form is written first. For example,
The e.m.f. of this cell is -0.76 V and the standard electrode potential of the Zn2+|Zn half cell is -0.76 V.
standard hydrogen electrode → standardna vodikova elektroda
Standard hydrogen electrode is a system in which hydrogen ion and gaseous hydrogen are present in their standard states. The convention is to designate the cell so that the standard hydrogen electrode is written first.
The electrode is used as a reference (of zero) for the values of other standard electrode potentials.
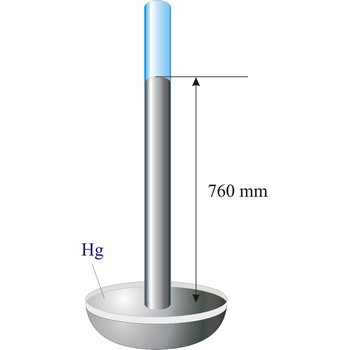
Torricelli, Evangelista → Torricelli, Evangelista
Evangelista Torricelli (1852-1908) is Italian physicist and mathematician. He became the first scientist to create a sustained vacuum and to discover the principle of a barometer. He filled a tube three feet long, and hermetically closed at one end, with mercury and set it vertically with the open end in a basin of mercury, taking care that no air-bubbles should get into the tube. The column of mercury invariably fell to about twenty-eight inches, leaving an empty space above its level. He discovered that the variation of the height of the mercury from day to day was caused by changes in the atmospheric pressure. He also constructed a number of large objectives and small, short focus, simple microscopes.
U-tube manometer → U-manometar
U-tube manometer contains water or mercury in a U-shaped tube, and is usually used to measure gas pressure. One end of the U tube is exposed to the unknown pressure field (P) and the other end is connected to a reference pressure source (usually atmospheric pressure) (Pref), shown in the schematic below.
If fluid C is the atmosphere, fluid B is the liquid in the U tube (e.g. water or mercury), and fluid A is a gas, then we can assume that ρB >> ρA, ρC. The pressure contributed by the weight of gas within the U tube can therefore be neglected. The gage pressure of the gas can be approximated by,
van der Waals’ equation → van der Waalsova jednadžba
Van der Waals’ equation is an equation of state for real fluids which takes the form:
where p is pressure, Vm is molar volume, T is temperature, R is the molar gas constant, and a and b are characteristic parameters of the substance which describe the effect of attractive and repulsive intermolecular forces.
Ziegler process → Zieglerov proces
Ziegler process is an industrial process for the manufacture of high-density polyethene using catalysts of titanium(IV) chloride (TiCl4) and aluminium alkyls (e.g. triethylaluminium, Al(C2H5)3). The process was introduced in 1953 by the German chemist Karl Ziegler (1898-1973). It allowed the manufacture of polythene at lower temperatures (about 60 °C) and pressures (about 1 atm) than used in the original process.
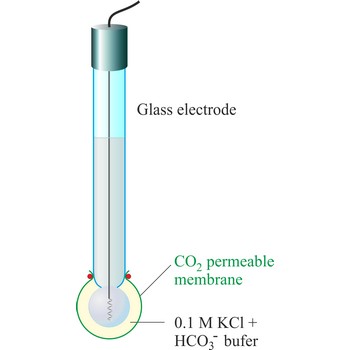
CO2 ion selective electrode → CO2 ion selektivna elektroda
The carbon dioxide ion selective electrode uses a gas-permeable membrane to separate the sample solution from the electrode internal solution. Dissolved carbon dioxide in the sample solution diffuses through the membrane until an equilibrium is reached between the partial pressure of CO2 in the sample solution and the CO2 in the internal filling solution. In any given sample the partial pressure of carbon dioxide will be proportional to the concentration of carbon dioxide. The diffusion across the membrane affects the level of hydrogen ions in the internal filling solution:
The hydrogen level of the internal filling solution is measured by the pH electrode located behind the membrane. The internal filling solution contains a high concentration of sodium bicarbonate (e.g. 0.1 mol/L NaHCO3) so that the bicarbonate level can be considered constant.
Citing this page:
Generalic, Eni. "Kritični tlak." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table