polonium → polonij
Polonium was discovered by Marie Curie (Poland) in 1898. Named for Poland, native country of Marie Curie. It is silvery-grey, extremely rare, radioactive metal. Soluble in dilute acids. Highly toxic. Severe radiotoxicity. Carcinogen. Polonium occurs in pitchblende. Produced by bombarding bismuth with neutrons. Used in industrial equipment that eliminates static electricity caused by such processes as rolling paper, wire and sheet metal.
praseodymium → praseodimij
Praseodymium was discovered by Carl F. Auer von Welsbach (Austria) in 1885. The origin of the name comes from the Greek words prasios didymos meaning green twin. It is silvery white, moderately soft, malleable, ductile metal. Reacts slowly with oxygen. Reacts rapidly with water. Metal ignites and burns readily. Praseodymium is obtained from same salts as neodymium. Used with neodymium to make lenses for glass maker’s goggles since it filters out the yellow light present in glass blowing. Alloyed with magnesium creates a high-strength metal used in aircraft engines. Misch metal, used in the manufacture of pyrophoric alloys for cigarette lighters, contains about 5 % praseodymium metal. (Typically composition of misch metal are Ce:Nd:Pr:La:Other rare earth=50:18:6:22:4).
proline → prolin
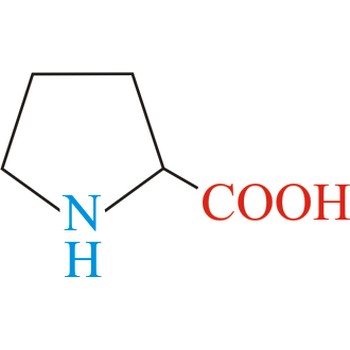
Proline has an aliphatic side chain with a distinctive cyclic structure. It is unusual because it is conformationally restricted. The secondary amino (imino) group of proline residues is held in a rigid conformation that reduces the structural flexibility of polypeptide regions containing proline. It is not an essential amino acid, which means that the human body can synthesize it.
- Abbreviations: Pro, P
- IUPAC name: pyrrolidine-2-carboxylic acid
- Molecular formula: C5H9NO2
- Molecular weight: 115.13 g/mol
protactinium → protaktinij
Protactinium was discovered by Otto Hahn (Germany) and Lise Meitner (Austria) in 1917. The origin of the name comes from the Greek word protos meaning first. It is very rare, silvery-white, extremely radioactive metal. Resists alkalis; reacts with oxygen and acids. Attacked by steam. Highly radiotoxic. Protactinium is extremely toxic and must be handled with great care. Protactinium does not occur in nature. Found among fission products of uranium, thorium and plutonium.
relative molecular mass → relativna molekularna masa
Relative molecular mass (Mr) is the ratio of the average mass per molecule or specified entity of a substance to 1/12 of the mass of nuclide 12C. Also called molecular weight. It is equal to the sum of the relative atomic masses of all the atoms that comprise a molecule. For example
Mr(H2SO4) = 2·Ar(H) + Ar(S) + 4·Ar(O)
= 2·1.0079 + 32.066 + 4·15.999
= 2.0158 + 32.066 + 63.996
= 98.078
rhenium → renij
Rhenium was discovered by Walter Noddack, Ida Tacke and Otto Berg (Germany) in 1925. The origin of the name comes from the Latin word Rhenus meaning river Rhine. It is rare and costly, dense, silvery-white metal. Tarnishes in moist air. Resists corrosion and oxidation. Dissolves in nitric and sulfuric acids. Has a very high melting point. Rhenium is found in small amounts in gadolinite and molybdenite. Mixed with tungsten or platinum to make filaments for mass spectrographs. Its main value is as a trace alloying agent for hardening metal components that are subjected to continuous frictional forces.
rhodium → rodij
Rhodium was discovered by William Hyde Wollaston (England) in 1804. The origin of the name comes from the Greek word rhodon meaning rose. It is hard, silvery-white metal. Inert in air and acids. Reacts with fused alkalis. Rhodium is obtained as a by-product of nickel production. Used as a coating to prevent wear on high quality science equipment and with platinum to make thermocouples.
ruthenium → rutenij
Ruthenium was discovered by Karl Karlovich Klaus (Russia) in 1844. The origin of the name comes from the Latin word Ruthenia meaning Russia. It is rare, extremely brittle, silver-grey metal. Unaffected by air, water or acids. Reacts with very hot (molten) alkalis. Ruthenium is found in pentlandite and pyroxinite. Used to harden platinum and palladium. Aircraft magnetos use platinum alloy with 10 % ruthenium.
selenium → selenij
Selenium was discovered by Jöns Jakob Berzelius (Sweden) in 1817. The origin of the name comes from the Greek word selene meaning moon. It is soft metalloid similar to sulfur. Ranges from grey metallic to red glassy appearance. Unaffected by water. Soluble in alkalis and nitric acid. Burns in air. Toxic by inhalation or ingestion. Selenium is obtained from lead, copper and nickel refining. Conducts electricity when struck by light. Light causes it to conduct electricity more easily. It is used in photoelectric cells, TV cameras, xerography machines and as a semiconductor in solar batteries and rectifiers. Also colours glass red.
Citing this page:
Generalic, Eni. "Kiselo-bazna titracija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table