acid-base titration → kiselo-bazna titracija
Acid-base titration is an analytical technique in volumetric analysis, where an acid of known concentration is used to neutralise a known volume of a base, and the observed volume of the acid required is used to determine the unknown concentration of the base. An acid-base indicator is used to determine the end-point of the titration.
acid-base indicator → kiselo-bazni indikator
Acid-base indicator is a weak acid or weak base, such as litmus, methyl orange or phenolphthalein, which changes colour when it gains or loses an H+ ion.
potentiometric titration → potenciometrijska titracija
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
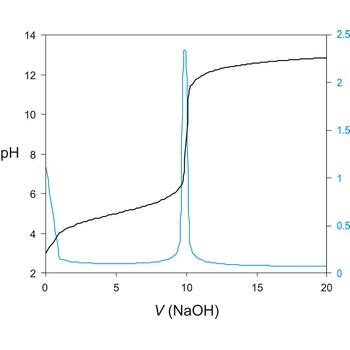
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
redox titration → redoks-titracija
Redox titration (oxidation-reduction titration) is a titration based on a redox reaction. For example, iron in water can be determined by converting dissolved iron to Fe2+ and titrating the solution with potassium permanganate (KMnO4), a powerful oxidising agent.
acid → kiselina
Acid is a type of compound that contains hydrogen and dissociates in water to produce positive hydrogen ions. The reaction for an acid HA is commonly written:
In fact, the hydrogen ion (the proton) is solvated, and the complete reaction is:
This definition of acids comes from the Arrhenius theory. Such acids tend to be corrosive substances with a sharp taste, which turn litmus red and produce colour changes with other indicators. They are referred to as protonic acids and are classified into strong acids, which are almost completely dissociated in water, (e.g. sulphuric acid and hydrochloric acid), and weak acids, which are only partially dissociated (e.g. acetic acid and hydrogen sulphide). The strength of an acid depends on the extent to which it dissociates, and is measured by its dissociation constant.
In the Lowry-Brønsted theory of acids and bases (1923), the definition was extended to one in which an acid is a proton donor (a Brønsted acid), and a base is a proton acceptor (a Brønsted base). An important feature of the Lowry-Brønsted concept is that when an acid gives up a proton, a conjugate base is formed that is capable of accepting a proton.
Similarly, every base produces its conjugate acid as a result of accepting a proton.
For example, acetate ion is the conjugate base of acetic acid, and ammonium ion is the conjugate acid of ammonia.
As the acid of a conjugate acid/base pair becomes weaker, its conjugate base becomes stronger and vice versa.
A further extension of the idea of acids and bases was made in the Lewis theory. In this, a G. N. Lewis acid is a compound or atom that can accept a pair of electrons and a Lewis base is one that can donate an electron pair. This definition encompasses "traditional" acid-base reactions, but it also includes reactions that do not involve ions, e.g.
in which NH3 is the base (donor) and BCl3 the acid (acceptor).
indicator → indikator
Indicator is a substance used to show the presence of a chemical substance or ion by its colour. Acid-base indicators are compounds, such as phenolphtaleine and methyl orange, which change colour reversibly, depending on whether the solution is acidic or basic. Oxidation-reduction indicators are substances that show a reversible colour change between oxidised and reduced forms.
methyl orange → metil oranž
Methyl orange is an acid-base indicator, in acid it turns red and in a base it turns yellow.
acid dissociation constant → konstanta disocijacije kiseline
Acid dissociation constant (Ka) is the equilibrium constant for the dissociation of an acid HA through the reaction
The quantity pKa = -log Ka is often used to express the acid dissociation constant.
acid halide → kiselinski halogenid
Acid halide is organic compound containing the group -COX where X is a halogen atom.
acid radical → kiseli radikal
Acid radical is an anion left after removal of hydrogen atoms from an acid.
Citing this page:
Generalic, Eni. "Kiselo-bazna titracija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table