chiral molecule → kiralne molekule
Chiral molecule is a molecule which cannot be superimposed on its mirror image. A common example is an organic molecule containing a carbon atom to which four different atoms or groups are attached. Such molecules exhibit optical activity, i.e., they rotate the plane of a polarised light beam.
linear molecular geometry → linearna geometrija molekule
Linear molecule is a molecule in which atoms are deployed in a straight line (under 180° angle). Molecules with an linear electron pair geometries have sp hybridization at the central atom. An example of linear electron pair and molecular geometry are carbon dioxide (O=C=O) and beryllium hydride BeH2.
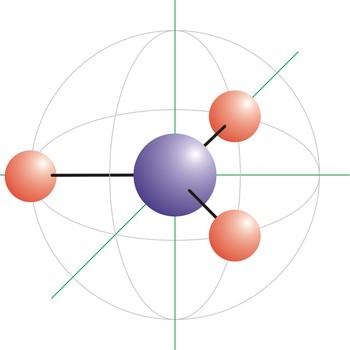
molecular shape → oblik molekule
Molecular shape is the three dimensional arrangement of atoms in space around a central atom. The molecular formula of a substance does not give an indication of its shape. For example, CO2 is a linear molecule, but SO2 is angular.
The three-dimensional shapes of many small molecules can be predicted by applying the valence shell electron pair repulsion theory (VSEPR). When atoms combine to form molecules, pairs of valence electrons arrange themselves as far from each other as possible. Another way to characterize molecular shape is in terms of hybrid orbitals.
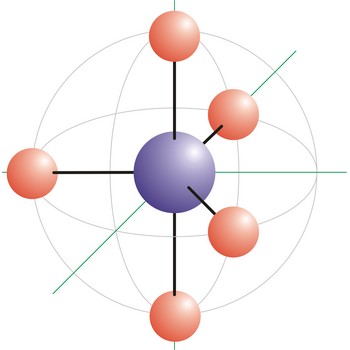
octahedral molecular geometry → oktaedarska geometrija molekule
Octahedral molecular geometry (square bipyramidal shape) describes the shape of compounds where six atoms or ligands are symmetrically arranged around a central atom. The sulfur hexafluoride (SF6), with six bonding pairs, is predicted and found to be a regular octahedron. Four of the attachments are positioned in a square plane with 90° bond angles. The remaining two attachments are positioned perpendicular (90°) to the square plane at opposite ends of the central atom. Molecules with an octahedral electron pair geometries have sp3d2 (or d2sp3) hybridization at the central atom.
planary structure → planarna struktura molekule
Planary structure of molecule is a structure of molecule in which all atoms in the molecule lie in the same plane.
square planar molecular geometry → kvadratna planarna geometrija molekule
Square planar is a molecular shape that results when there are four bonds and two lone pairs on the central atom in the molecule. An example of a square planar molecule is xenon tetrafluoride (XeF4). This molecule is made up of six equally spaced sp3d2 (or d2sp3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. Two orbitals contain lone pairs of electrons on opposite sides of the central atom. The remaining four atoms connected to the central atom give the molecule a square planar shape.
square pyramidal molecular geometry → kvadratna piramidalna geometrija molekule
Square pyramidal is a molecular shape that results when there are five bonds and one lone pair on the central atom in the molecule. Bromine pentafluoride (BrF5) has the geometry of a square pyramid, with fluorine atoms occupying five vertices, one of which is above the plane of the other four. This molecule is made up of six equally spaced sp3d2 (or d2sp3) hybrid orbitals arranged at 90° angles. The shape of the orbitals is octahedral. Because of the high symmetry of the octahedral arrangement, all six positions are equivalent, so it does not matter in which position in the drawing we put the lone pair. The remaining four atoms connected to the central atom give the molecule a square planar shape.
T-shaped molecular geometry → T-oblik geometrije molekule
T-shape is a molecular geometry that results when there are 3 bonds and 2 lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the ends of a T with 90° angles between them. Molecules with an trigonal bipyramidal electron pair geometries have sp3d (or dsp3) hybridization at the central atom. ICl3 has a T-shaped molecular geometry.
tetrahedral molecular geometry → tetraedarska geometrija molekule
Tetrahedral is a molecular shape that results when there are four bonds and no lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the corners of a tetrahedron with 109.5° angles between them. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom. The ammonium ion (NH4+) and methane (CH4) have a tetrahedral molecular geometry.
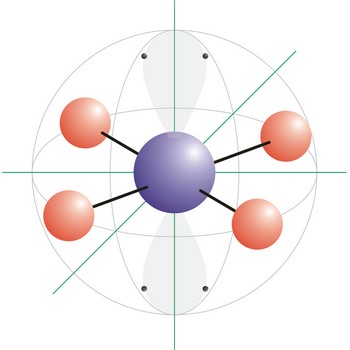
trigonal bipyramidal molecular geometry → trigonska bipiramidalna geometrija molekule
Trigonal bipyramidal (trigonal bipyramidal shape) is a molecular geometry that results when there are five bonds and no lone pairs on the central atom in the molecule. Three of the bonds are arranged along the atom’s equator, with 120° angles between them; the other two are placed at the atom’s axis. Axial bonds are at right angles to the equatorial bonds. Molecules with an trigonal bipyramidal electron pair geometries have sp3d (or dsp3) hybridization at the central atom. The PCl5 molecule has a trigonal bipyramidal molecular geometry.
Citing this page:
Generalic, Eni. "Kiralne molekule." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table