valence bond theory → teorija valentne veze
Valence bond theory is a theory that explains the shapes of molecules in terms of overlaps between half-filled atomic orbitals, or half filled hybridised orbitals.
ion-product constant → ionski produkt vode
The ion-product constant. For the reaction:
the equilibrium expression would be:
Note that all pure liquid terms are omitted, hence H2O does not appear in the denominator. At 25 °C
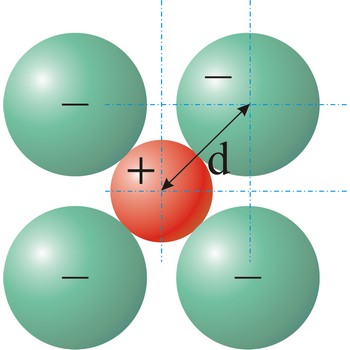
ionic radius → ionski radijus
Ionic radius is the radius of anions and cations in crystalline ionic compounds, as determined by consistently partitioning the center-to-center distance of ions in those compounds. In general, negative ions have larger ionic radii than positive ions.
water ion product → ionski produkt vode
Water ion product (Kw) is a concentration product of hydrogen and hydroxide ions. For the reaction:
the equilibrium expression would be:
Note that all pure liquid terms are omitted, hence H2O does not appear in the denominator. At 25 °C, Kw = 1.0×10-14 mol2dm-6 = (Ka)(Kb)
atom radius → radijus atoma
Atoms and molecules have no strict boundaries. The volume of a free atom is usually defined as that volume that contains 90 % of electron cloud. The radius of an atom represents half of interatom distance of two identical atoms which are in touch but are not interconnected either by a covalent or an ionic bond, but with a very weak van der Waals’s bond.
electronegativity → elektronegativnost
Electronegativity is a parameter originally introduced by L. Pauling which describes, on a relative basis, the power of an atom to attract electrons. For example, in hydrogen chloride, the chlorine atom is more electronegative than the hydrogen and the molecule is polar, with a negative charge on the chlorine atom.
There are various ways of assigning values for the electronegativity of an element. Pauling electronegativities are based on bond dissociation energies using a scale in which fluorine, the most electronegative element, has the value 4 and francium, the lowest electronegative element, has the value 0.7.
Fajans’ rules → Fajansova pravila
Fajans’ rules, formulated by American chemist of Polish origin. Kazimierz Fajans (1887-1975), indicating the extent to which an ionic bond has covalent character caused by polarisation of the ions. Covalent character is more likely if:
1. the charge of the ions is high;
2. the positive ion is small or the negative ion is large;
3. the positive ion has an outer electron configuration that is not a noble- gas configuration.
activity coefficient → koeficijent aktiviteta
Activity coefficient (γ or f) is a fractional number which, when multiplied by the molar concentration of a substance in solution, yields the chemical activity. This term gives an idea of how much interaction exists between molecules at higher concentration.
In solutions of very low ionic strength, when m is less than 0.01, the Debye-Hückel limiting law can be used to calculate approximate activity coefficients
where γi = activity coefficient of the species i, zi = charge on the species i and μ = ionic strength of the solution.
Citing this page:
Generalic, Eni. "Ionska veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table