close-packed structure → gusta slagalina
Close packing is the packing of spheres so as to occupy the minimum amount of space. The name close packed refers to the packing efficiency of 74.05 %. There are two types of close packing: hexagonal and cubic. One layer, with atoms centered on sites labeled a. Two layers, with the atoms of the second layer centered on sites labeled b. The third layer can be placed on the sites labeled c (giving cubic close-packing) or over those marked a (giving hexagonal close-packing).
colloid → koloid
Colloids are systems in which there are two or more phases, with one (the dispersed phase) distributed in the other (the continuous phase). Moreover, at least one of the phases has small dimensions, in the range between 1 nm and 1 μm (10-9 m – 10-6 m). Dimension, rather than the nature of the material, is characteristic. In this size range, the surface area of the particle is large with respect to its volume so that unusual phenomena occur, e.g., the particles do not settle out of the suspension by gravity and are small enough to pass through filter membranes. Macromolecules (proteins and other high polymers) are at the lower limit of this range; the upper limit is usually taken to be the point at which the particles can be resolved in an optical microscope.
Colloidal particles may be gaseous, liquid, or solid, and occur in various types of suspensions:
Sols - dispersions of small solid particles in a liquid.
Emulsions - colloidal systems in which the dispersed and continuous phases are both liquids.
Gels - colloids in which both dispersed and continuous phases have a three-dimensional network throughout the material.
Aerosols - colloidal dispersions of liquid or solid particles in a gas.
Foams - dispersions of gases in liquids or solids.
free energy → slobodna energija
Free energy is an energy that is actually available to do useful work. A decrease in free energy accompanies any spontaneous process. Free energy does not change for systems that are at equilibrium.
condensation → kondenzacija
1. Condensation is a process of changing from a gaseous to a liquid or solid state, usually done by cooling.
2. Condensation, in colloid systems, is a process where smaller particle join in one colloid size particle
3. Condensation, in chemical terms, is a sort of chemical reaction in which small molecules like water, carbon dioxide, or ammonia single out.
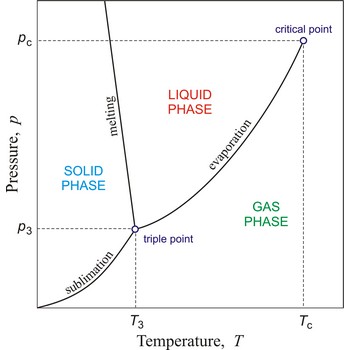
critical point → kritična točka
In general, critical point is the point on the phase diagram of a two-phase system at which the two coexisting phases have identical properties and therefore represent a single phase. At the liquid-gas critical point of a pure substance, the distinction between liquid and gas vanishes, and the vapour pressure curve ends. The coordinates of this point are called the critical temperature and critical pressure. Above the critical temperature it is not possible to liquefy the substance.
degree → stupanj
1. Degree is a unit of temperature on a specified scale. The temperatures of boiling and freezing water are: in the Fahrenheit system 212 and 32 degrees; in the Celsius system 100 and 0 (zero) degrees.
2. Degree is a unit of angular measure. A circle is divided into 360 degrees, represented by the symbol °. Degrees are each divided into 60 minutes. Each minute has 60 seconds. Symbols for degree, minute, and second for plane angle is placed after the numerical value and a no space between the numerical value and the unit symbol (α = 2°3'4").
3. In algebra, the degree of a polynomial is the highest power of the variable in the polynomial. For example, 4x3 + 3x2 + x + 7 have degree 3.
glacial acetic acid → ledena octena kiselina
Glacial acetic acid (CH3COOH) is the pure compound, as distinguished from the usual water solutions known as acetic acid. It is a colorless liquid or crystalline substance (melting point 16.6 °C) with a pungent, vinegar odor.
hardening → stvrdnjavanje
Hardening is the process of becoming hard or solid by cooling, or drying, or crystallization. For example, the hardening of concrete.
Citing this page:
Generalic, Eni. "Hexagonal_crystal_system." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table