ideal crystal → idealni kristal
Ideal crystal is a single crystal with a perfectly regular lattice that contains no impurities, imperfections, or other defects.
isotropic crystal → izotropni kristal
The measured properties of an isotropic crystal are independent of the axis of testing. Opposite of anisotropic.
liquid crystal → tekući kristal
Liquid crystals or crystalline liquids are a physical state between crystals and melts. The liquid crystalline phase - the so-called mesophase - is formed at the melting point. The most important (usable) mesophases are nematic, cholesteric and smectic phase, having different molecular orientations.
groups in periodic system of elements → skupine periodnog sustava
Periodic system of elements is divided into 18 groups of chemical elements. Elements belonging to the same group have a same number of valence electrons and similar chemical properties. Elements of main groups are in 1., 2., and in groups 13. to 18. Different groups of elements can be named according to the first element in the group (elements of boron group, elements of carbon group), or they have some special names (noble gases, halogenic elements, halyde elements, earthalkali and alkali metals).
open system → otvoreni sustav
Open system is a system which can exchange matter and energy with the environment.
perfect crystal → savršeni kristal
Perfect crystal is a crystal with no defects or impurities made of completely identical repeating subunits. Further, a perfect crystal has only one possible arrangement of subunits, with every subunit making exactly the same contribution to the total energy of the crystal.
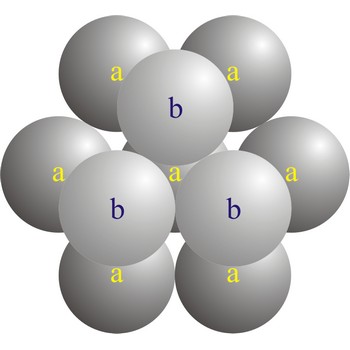
hexagonal close-packed structure → heksagonska gusta slagalina
In a hexagonal close-packed (hcp) arrangement of atoms, the unit cell consists of three layers of atoms. The top and bottom layers (a) contain six atoms at the corners of a hexagon and one atom at the center of each hexagon. The middle layer (b) contains three atoms nestled between the atoms of the top and bottom layers, hence, the name close-packed. The hexagonal close packed structure can be made by piling layers in the a-b-a-b-a-b... sequence.
hexagonal lattice → heksagonska rešetka
Hexagonal lattice has lattice points at the twelve corners of the hexagonal prism and at the centers of the two hexagonal faces of the unit cell. It has unit cell vectors a=b≠c and interaxial angles α=β=90° and γ=120°.
international system of units → međunarodni sustav jedinica
International System of Units (SI) is the unit system adopted by the General Conference on Weights and Measures in 1960 and recommended for use in all scientific and technical fields. It consists of seven base units (meter, kilogram, second, ampere, kelvin, mole, candela), plus derived units and prefixes.
system → sustav
System is the region under consideration, as distinguished from the rest of the universe (the environment). Systems may be separated from environments by boundaries that prevent the transfer of mass (a closed system), of heat (an adiabatic system), or of any energy (an isolated system). Systems that exchange mass with the environment are open systems.
Citing this page:
Generalic, Eni. "Hexagonal_crystal_system." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table