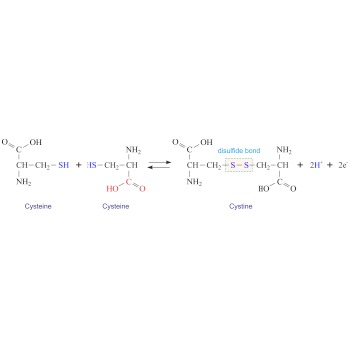
Cystine → Cistin
Cystine (C6H12N2O4S2) is a dimer of cysteine. It is formed by the oxidation of the thiol groups (-SH) of two cysteines generating a disulphide bridge (-S-S-). Cystine is a white crystalline solid that is slightly soluble in water. Cystine is particularly abundant in skeletal and connective tissues and in hair, horn, and wool.
Heyrovsky-Ilkovic equation → Heyrovsky-Ilkovičeva jednadžba
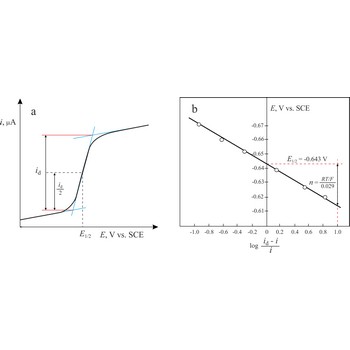
The Heyrovsky-Ilkovic equation describes the entire current-potential curve (polarographic wave) of a reversible redox system in polarography
where R is the gas constant, T is the absolute temperature, F is the Faraday constant, n denotes the number of electrons taking part in the electrode reaction. E1/2 is a unique potential (for a given reaction and supporting electrolyte) termed the half-wave potential.
In order to obtain E1/2 from the above equation, we plot a graph of ln[(id-i)/i] against E. The intercept on the x-axis gives then an accurate value of E1/2. The slope of the obtained straight line is equal to nF/RT from which n is determined.
Citing this page:
Generalic, Eni. "Hexagonal_crystal_system." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table