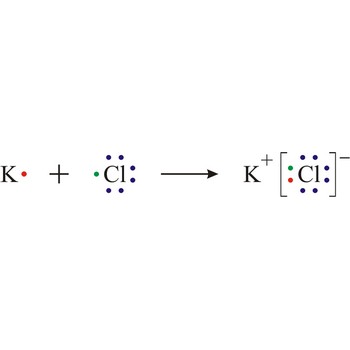solid solution → čvrste otopine
Solid solution is a crystalline material that is a mixture of two or more components, with ions, atoms, or molecules of one component replacing some of the ions, atoms of the other component in its normal crystal lattice.
ionic bond → ionska veza
Ionic bond is a strong force of attraction holding atoms together in a molecule or crystal. Typically chemical bonds have energies of about 100 kJ mol-1. Ionic bond is a bond at which one of the participants, during the procedure of bonding, gives away its unpaired electrons to another atom so that both can achieve electron arrangement of the closest noble gas. In order to form an ionic bond one of the atoms must cross to the positively charged ion by losing certain number of electrons and the other atom must receive those electrons and cross to the negatively charged ion.
ionic radius → ionski radijus
Ionic radius is the radius of anions and cations in crystalline ionic compounds, as determined by consistently partitioning the center-to-center distance of ions in those compounds. In general, negative ions have larger ionic radii than positive ions.
ionisation → ionizacija
Ionisation is the process of producing ions. Certain molecules ionise in a solution; for example, acids ionise when dissolved in water.
Electron transfer also causes ionisation in certain reactions, for example sodium and chlorine react by transfer of a valence electron from the sodium atom to the chlorine atom to form the ions that constitute a sodium chloride crystal.
Joule-Thomson’s effect → Joule-Thomsonov efekt
Temperature of ideal gas will not be changed when it is repressed to a lower pressure, but when real gases are repressed to a lower pressure, a lower or higher temperature change appears under high pressures. The temperature change which appears at real gas expansion in a system into which energy is not brought is called Joule-Thomson’s effect. It was determined that when air is repressed by 1 bar, its temperature drops by 0.25 °C. That minute effect is completely irrelevant for most technical processes, but is also used in gas liquefying procedure.
thermodynamic equilibrium → termodinmička ravnoteža
Thermodynamic equilibrium is a system equilibrium in which energy that it gains from its surroundings is exactly balanced by the energy that it loses, no matter how much time is allowed to pass.
metal → metal
Metals are materials in which the highest occupied energy band (conduction band) is only partially filled with electrons.
Their physical properties generally include:
- They are good conductors of heat and electricity. The electrical conductivity of metals generally decreases with temperature.
- They are malleable and ductile in their solid state.
- They show metallic lustre.
- They are opaque.
- They have high density.
- They are solids (except mercury)
- They have a crystal structure in which each atom is surrounded by eight to twelve near neighbours
Their chemical properties generally are:
- They have one to four valence electrons.
- They have low ionisation potentials; they readily lose electrons.
- They are good reducing agents.
- They have hydroxides which are bases or amphoteric.
- They are electropositive.
Metallic characteristics of the elements decrease and non-metallic characteristics increase with the increase of valence electrons. Also metallic characteristics increase with the number of electron shells. Therefore, there is no sharp dividing line between the metals and non-metals.
Of the 114 elements now known, only 17 show primarily non-metallic characteristics, 7 others are metalloids, and 89 may be classed as metals.
metallic glass → metalno staklo
Certain alloys can solidify by extremely rapid cooling out of melt without formation of a crystal lattice, that is in the amorphous form - such, amorphous alloys are so called metallic glasses. The alloy of zirconium, beryllium, titanium, copper, and nickel is one of the first metallic glasses that can be made in bulk and formed into strong, hard, useful objects.
Unlike pure metals and most metal alloys, metallic glasses have no regular crystalline structure. This lack of long range order or microstructure is related to such desirable features as strength and low damping which is one reason why the premier use for zirconium-based metallic glass is in the manufacture of expensive golf club heads. Metallic glasses can be quite strong yet highly elastic, and they can also be quite tough (resistant to fracture). Even more interesting are the thermal properties; for instance, just like an oxide glass, there is a temperature (called the glass transition temperature) above which a metallic glass becomes quite soft and flows easily. This means that there are lots of opportunities for easily forming metallic glasses into complex shapes.
transitional temperature → prijelazna temperatura
Transitional temperature is a temperature at which the crossing from one kind of crystals to another appears to be of the same matter (polymorphy).
Citing this page:
Generalic, Eni. "Hexagonal_crystal_system." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table