carbon → ugljik
Carbon has been known since ancient times. The origin of the name comes from the Latin word carbo meaning charcoal. Graphite form of carbon is a black, odourless, slippery solid. Graphite sublimes at 3825 °C. Diamond form is a clear or colored; an extremely hard solid. C60 is Buckminsterfullerine. Carbon black burns readily with oxidants. Carbon is made by burning organic compounds with insufficient oxygen. There are close to ten million known carbon compounds, many thousands of which are vital to organic and life processes. Radiocarbon dating uses the carbon-14 isotope to date old objects.
cement → cement
Cement is any various substances used for bonding or setting to a hard material. Portland cement is a mixture of calcium silicates and aluminates made by heating limestone (CaCO3) with clay (containing aluminosilicates) in a kiln. The product is ground to a fine powder. When mixed with water it settles in a few hours and then hardens over a longer period of time due to the formation of hydrated aluminates and silicates.
Lewis, Gilbert N. → Lewis, Gilbert N.
Gilbert Newton Lewis (1875-1946) is an American chemist whose theory of the electron pair fostered understanding of the covalent bond and extended the concept of acids and bases.
Markovnikov’s rule → Markovnikovljevo pravilo
Markovnikov’s rule: when an asymmetrical alkene reacts with a hydrogen halide to give an alkyl halide, the hydrogen adds to the carbon of the alkene that has the greater number of hydrogen substituents, and the halogen to the carbon of the alkene with the fewer number of hydrogen substituents.
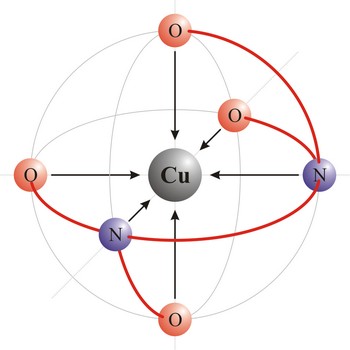
chelate → kelat
Chelate is a compound characterized by the presence of bonds from two or more bonding sites within the same ligand to a central metal atom. For example, copper complexes with EDTA to form a chelate. Chelate complexes are more stable than complexes with the corresponding monodentate ligands.
chiral molecule → kiralne molekule
Chiral molecule is a molecule which cannot be superimposed on its mirror image. A common example is an organic molecule containing a carbon atom to which four different atoms or groups are attached. Such molecules exhibit optical activity, i.e., they rotate the plane of a polarised light beam.
collision theory → teorija sudara
Collision theory is theory that explains how chemical reactions take place and why rates of reaction alter. For a reaction to occur the reactant particles must collide. Only a certain fraction of the total collisions cause chemical change; these are called successful collisions. The successful collisions have sufficient energy (activation energy) at the moment of impact to break the existing bonds and form new bonds, resulting in the products of the reaction. Increasing the concentration of the reactants and raising the temperature bring about more collisions and therefore more successful collisions, increasing the rate of reaction.
conformation → konformacija
Conformation is one of the very large numbers of possible spatial arrangements of atoms that can be interconverted by rotation about a single bond in a molecule. The conformation of a molecule is not fixed, though one or another shape may be more likely to occur. There are two extreme cases:
Staggered conformation (antiperiplanar) is a conformation about a carbon-carbon single bond in which the atoms on one carbon are as far apart as possible from the atoms on an adjacent carbon.
Eclipsed conformation (syn-periplanar) is a conformation about a carbon-carbon single bond in which the atoms on one carbon are as close as possible to the atoms on an adjacent carbon.
monodentate ligand → monodentantni ligand
Monodentate ligand is a ligand that has only one atom that coordinates directly to the central atom in a complex. For example, ammonia and chloride ion are monodentate ligands of copper in the complexes [Cu(NH3)6]2+ and [CuCl6]2+.
Citing this page:
Generalic, Eni. "Glikozidna veza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table