change of state → promjena stanja
Change of state is a physical change which appears when a substance crosses from one state into another. This usually happens because of the change of energy of particles provoked by heating or cooling.
chemical potential → kemijski potencijal
For a mixture of substances, the chemical potential of constituent B (μB) is defined as the partial derivative of the Gibbs energy G with respect to the amount (number of moles) of B, with temperature, pressure, and amounts of all other constituents held constant.
Also called partial molar Gibbs energy. Components are in equilibrium if their chemical potentials are equal.
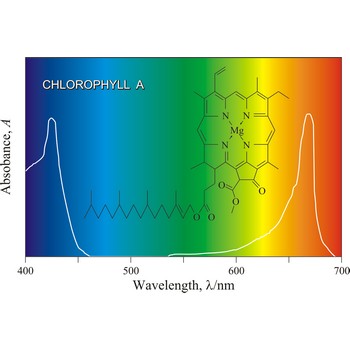
chlorophyll → klorofil
Chlorophyll is a green pigment present in green plants and cyanobacteria. Chlorophyll is essential in the transformation of light energy to chemical energy in photosynthesis. Chlorophyll absorbs light mostly in the blue and red ends of the visible spectrum, and very little in the green wavelengths. That green light is reflected, giving us the leaf colour we see.
irreversible process → ireverzibilni proces
If a system is taken from one state to another but cannot be brought back to the same initial state, then the process is called irreversible. Some examples are free expansion of a gas; dissipation of energy due to friction, or the mixing of two gases or liquids etc.
joule → džul
Joule (J) is the SI derived unit of energy, work, and heat. The joule is the work done when the point of application of a force of one newton is displaced a distance of one metre in the direction of the force (J = N m). The unit was named after the British scientist James Prescott Joule (1818-1889).
density → gustoća
In the most common usage, density (ρ) is mass density or mass per unit volume. In Si units it is measured in kg m-3. More commonly, densities are given in kg dm-3.
More generally, it is the amount of some quantity (mass, charge, energy, etc.) divided by a length, area, or volume.
Relative density is the ratio of the density of a substance to the density of some reference substance. For liquids or solids, it is the ratio of the density (usually at 20 °C) to the density of water at 4 °C. This quantity was formerly called specific gravity.
dissociation → disocijacija
Dissociation is the process by which a chemical combination breaks up into simpler constituents as a result of either added energy (dissociated by heat), or the effect of a solvent on a dissolved polar compound (electrolytic dissociation). It may occur in the gaseous, solid, or liquid state, or in a solution.
An example of dissociation is the reversible reaction of hydrogen iodide at high temperatures
The term dissociation is also applied to ionisation reactions of acids and bases in water. For example
which is often regarded as a straightforward dissociation into ions
ecological footprint → ekološki otisak
The Ecological Footprint is defined as the area of productive land and water ecosystems required to produce the resources that the population consumes (food, fiber, timber, energy, and space for infrastructure) and assimilate the wastes that the population produces (CO2 is the only waste product currently included), wherever on Earth the land and water is located. It compares actual throughput of renewable resources relative to what is annually renewed. Non-renewable resources are not assessed, as by definition their use is not sustainable.
Ecological footprints and biocapacity are expressed in global hectares (gha). Each unit corresponds to one hectare of biologically productive space with world average productivity. In U.S. Footprint results are often presented in global acres (ga). One U.S. acre is equal to 0.405 hectares.
Humanity is currently consuming renewable resources at a faster rate than ecosystems can regenerate them and continuing to release more CO2 than ecosystems can absorb. In 2007, humanity's Footprint was 18 billion gha, or 2.7 gha per person. However, the Earth's biocapacity was only 11.9 billion gha, or 1.8 gha per person. This represents an ecological overshoot of 50 per cent. Put another way, people used the equivalent of 1.5 planets to support their activities (more developed countries generally make higher demands on the Earth's ecosystems than poorer, less developed countries).
metabolism → metabolizam
Metabolism is a sum of all chemical and physiological processes by which the body builds and maintains itself. It is a process of building the body’s molecular structures from nutrients (anabolism) and breaking them down for energy (catabolism).
Einstein equation → Einsteinova jednadžba
Einstein equation is the mass-energy relationship introduced by Albert Einstein in 1905 in the form E = mc2, where E is a quantity of energy, m its mass, and c is the speed of light. It presents the concept that energy possesses mass.
Citing this page:
Generalic, Eni. "Energija aktivacije." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table