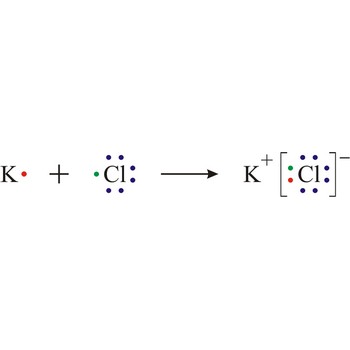transumutation → transmutacija
Transmutation is the conversion of one element into another by a process taking place in the nucleus.
X-ray spectrum → spektar X-zraka
X-ray spectrum is a set of characteristic X-ray frequencies or wavelengths produced by a substance used as a target in an X-ray tube. Each element has a characteristic X-ray spectrum, and there is a strong correlation between atomic number and the frequencies of certain lines in the X-ray spectrum.
Lewis structure → Lewisova struktura
Lewis structure is the representation of the electron arrangement in atoms, ions, or molecules by showing the valence electrons as dots placed around the symbols for the elements.
lithium → litij
Lithium was discovered by Johan August Arfvedson (Sweden) in 1817. The origin of the name comes from the Greek word lithos meaning stone, apparently because it was discovered from a mineral source whereas the other two elements, sodium and potassium, were discovered from plant sources. It is soft silvery-white metal. Lightest of metals. Reacts slowly with water and oxygen. Flammable. Can ignite in air. Reacts with water to give off a flammable gas. Lithium is obtained by passing electric charge through melted lithium chloride and from the silicate mineral called spodumene [LiAl(Si2O6)]. Used in batteries. Also for certain kinds of glass and ceramics. Some is used in lubricants.
metal → metal
Metals are materials in which the highest occupied energy band (conduction band) is only partially filled with electrons.
Their physical properties generally include:
- They are good conductors of heat and electricity. The electrical conductivity of metals generally decreases with temperature.
- They are malleable and ductile in their solid state.
- They show metallic lustre.
- They are opaque.
- They have high density.
- They are solids (except mercury)
- They have a crystal structure in which each atom is surrounded by eight to twelve near neighbours
Their chemical properties generally are:
- They have one to four valence electrons.
- They have low ionisation potentials; they readily lose electrons.
- They are good reducing agents.
- They have hydroxides which are bases or amphoteric.
- They are electropositive.
Metallic characteristics of the elements decrease and non-metallic characteristics increase with the increase of valence electrons. Also metallic characteristics increase with the number of electron shells. Therefore, there is no sharp dividing line between the metals and non-metals.
Of the 114 elements now known, only 17 show primarily non-metallic characteristics, 7 others are metalloids, and 89 may be classed as metals.
mole → mol
Mole (mol) is the SI base unit of amount of substance.
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kg of carbon 12.
When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. In this definition, it is understood that the carbon 12 atoms are unbound, at rest and in their ground state.
Newton’s gravitational law → Newtonov zakon gravitacije
Every object in the universe attracts every other object with a force (gravitational force FG) directed along the line through centres of the two objects that is proportional to the product of their masses and inversely proportional to the square of the distance between them.
m1 and m2 are masses of the two objects and r is the distance between them. G is universal constant of gravitation, which equals 6.67•10-26 N m2 kg-2. Strictly speaking, this law applies only to objects that can be considered pointlike object. Otherwise, the force has to be found by integrating the forces between various mass elements.
It is more properly to express Newton’s gravitational law by vector equation:
in which r1 and r2 are position vectors of masses m1 and m2.
Gravitational forces act on distance. Newton’s gravitational law is derived from Kepler’s law for planetary motion, using a physical assumption considering Sun as the centre and the source of gravitational force.
Additionally, every object moves in the direction of the force acting on it, with acceleration that is inversely proportional to the mass of object. For bodies on the surface of Earth, the distance r in gravitational law formula is practically equal to the Earth radius, RE. If the mass of the body on Earth surface is m and the mass of earth is ME, the gravitational force acting on that body can be expressed as:
where g is gravitational acceleration which is, although dependent on geographical latitude, usually considered as constant equal to 9.81 m s-2.
noble gas → plemeniti plin
Noble gas refers to any element of the group of six elements in group 18 of the periodic table. They are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Unlike most elements, the noble gases are monoatomic. The atoms have stable configurations of electrons. Therefore, under normal conditions they do not form compounds with other elements.
They were generally called inert gases until about 1962 when xenon tetrafluoride, XeF4, was produced in the laboratory. This was the first report of a stable compound of a noble gas with another single element.
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
octet rule → pravilo okteta
Octet rule states that the chemical properties of the elements repeat on a regular basis with increasing atomic mass, and that the chemical properties of each eight element are similar. Since the inert gases, with the exception of helium have eight electrons in their outer shells, this stable electronic configuration is called the octet rule. In chemical reactions atoms of elements tend to react in such a way as to achieve the electronic configuration of the inert gas nearest to them in the periodic table. There are a number of exceptions to the octet rule.
Citing this page:
Generalic, Eni. "Elementi rijetkih zemalja." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table