ion selective electrode → ion selektivne elektrode
Ion selective electrode (ISE) is an electrode or electrode assembly with a potential that is dependent on the concentration of an ionic species in the test solution and is used for electroanalysis. Ion-selective electrodes are often membrane type electrodes.
redox potential → redoks potencijal
Redox potential is the potential of a reversible oxidation-reduction electrode measured with respect to a reference electrode, corrected to the hydrogen electrode, in a given electrolyte.
referent electrode → referentna elektroda
Referent electrode is an electrode whose potential is known and completely independent of analyte concentration. Mostly used referent electrodes are calomel and silver/silver chloride electrode.
Table: Dependence of referent electrodes potentials on KCl concentration
| Potential vs. SHE / V | |||||
| calomel electrode | Ag/AgCl electrode | ||||
| t / °C | 0.1 mol dm-3 | 3.5 mol dm-3 | sat. solution | 3.5 mol dm-3 | sat. solution |
| 15 | 0.3362 | 0.254 | 0.2511 | 0.212 | 0.209 |
| 20 | 0.3359 | 0.252 | 0.2479 | 0.208 | 0.204 |
| 25 | 0.3356 | 0.250 | 0.2444 | 0.205 | 0.199 |
| 30 | 0.3351 | 0.248 | 0.2411 | 0.201 | 0.194 |
| 35 | 0.3344 | 0.246 | 0.2376 | 0.197 | 0.189 |
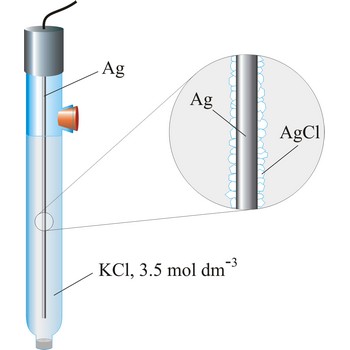
silver/silver-chloride electrode → srebro/srebrov klorid elektroda
Silver/silver-chloride electrode is by far the most common reference type used today because it is simple, inexpensive, very stable and non-toxic. It is mainly used with saturated potassium chloride electrolyte, but can be used with lower concentrations such as 3.5 mol dm-3 or 1 mol dm-3 potassium chloride. Silver/silver-chloride electrode is a referent electrode based on the following halfreaction
| Potential vs. SHE / V | ||
|---|---|---|
| t / °C | 3.5 mol dm-3 | sat. solution |
| 15 | 0.212 | 0.209 |
| 20 | 0.208 | 0.204 |
| 25 | 0.205 | 0.199 |
| 30 | 0.201 | 0.194 |
| 35 | 0.197 | 0.189 |
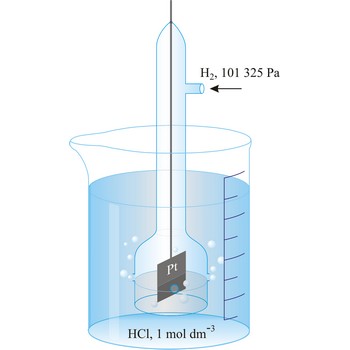
standard hydrogen electrode → standardna vodikova elektroda
Standard hydrogen electrode is a system in which hydrogen ion and gaseous hydrogen are present in their standard states. The convention is to designate the cell so that the standard hydrogen electrode is written first.
The electrode is used as a reference (of zero) for the values of other standard electrode potentials.
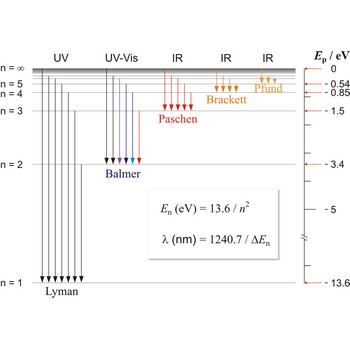
Balmer series → Balmerova serija
Balmer series, Balmer lines is a series of lines in the emission spectrum of hydrogen that involve transitions to the n=2 state from states with n>2.
decomposition potential → potencijal razlaganja
Decomposition potential of some system is the smallest voltage which should be applied so that electrolysis occurs.
Donnan potential → Donnanov potencijal
Donnan potential is the electrical potential difference between two solutions separated by an ion-exchange membrane in the absence of any current flowing through the membrane
electrochemical potential → elektrokemijski potencijal
Electrochemical potential is a measure of a metal to form ions in a liquid.
Citing this page:
Generalic, Eni. "Electrode potential series." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table