Results 1–3 of 3 for dijastereoizomer
diastereoisomer → dijastereoizomer
Diastereoisomers (diastereomers) are stereoisomers of a compound having two or more chiral centers that are not a mirror image of another stereoisomer of the same compound. For example, in the structure below, 1 and 2 are enantiomers and so are 3 and 4; 1 and 3 are diastereoisomers, as are 2 and 4. Unlike enantiomers, diastereoisomers need not have closely similar physical and chemical properties
anomer → anomer
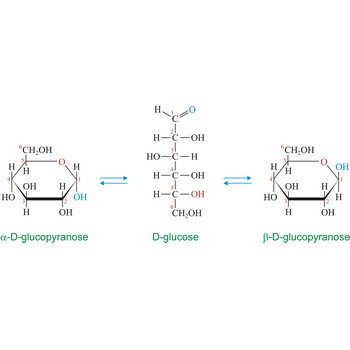
Anomers are diastereoisomers of cyclic forms of sugars or similar molecules differing in the configuration at the anomeric carbon (C-1 atom of an aldose or the C-2 atom of a 2-ketose). The cyclic forms of carbohydrates can exist in two forms, α- and β- based on the position of the substituent at the anomeric center. Anomer are designated α if the configuration at the anomeric carbon is the same as that at the reference asymmetric carbon in a Fischer projection. If the configuration differs the anomer is designated β. For example, α-D-glucopyranose and β-D-glucopyranose, the two cyclic forms of glucose, are anomers.
epimer → epimer
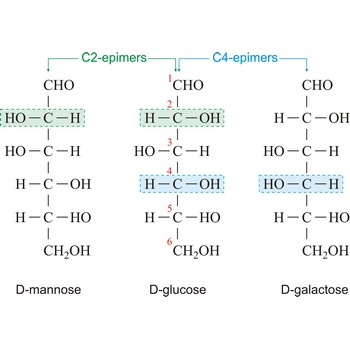
Epimers are diastereoisomers that have the opposite configuration at only one of two or more chiral centers present in the respective molecular entities. For example D-glucose and D-mannose, which differ only in the stereochemistry at C-2, are epimers, as are D-glucose and D-galactose (which differ at C-4).
Citing this page:
Generalic, Eni. "Dijastereoizomer." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table