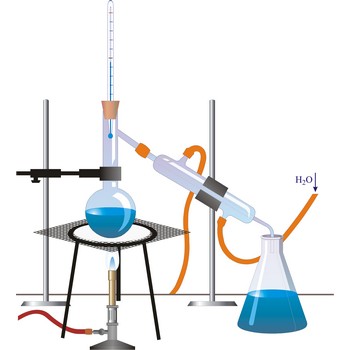
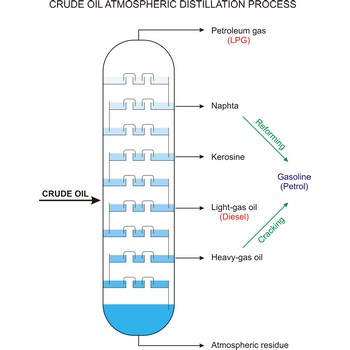
distillation → destilacija
Distillation is a process of boiling a liquid and condensing and collecting the vapour. The liquid collected is the distillate. The usual purpose of distillation is purification or separation of the components of a mixture. This is possible because the composition of the vapour is usually different from that of liquid mixture from which it is obtained. Petrol, kerosene, fuel oil, and lubricating oil are produced from petroleum by distillation.
vacuum distillation → vakuum destilacija
Vacuum distillation is distillation under reduced pressure. The depression in the boiling point of the substance distilled means that the temperature is lower, which may prevent the substance from decomposing.
fractional distillation → frakcijska destilacija
Fractional distillation is a procedure in which liquids of close boiling points are separated. It is conducted in fraction or rectification columns in a way that vapour phase created by distillation is condensed and the condensate thus obtained is redistilled. The procedure is repeated several times. Vapour phase always contains more volatile component than the liquid phase, at top of the column vapour of clean volatile component gets out and at the bottom of the column liquid of nonvolatile component.
argon → argon
Argon was discovered by Lord Raleigh and Sir William Ramsay (Scotland) in 1894. The origin of the name comes from the Greek word argos meaning inactive. It is colourless and odourless noble gas. Chemically inert. It is the third most abundant element in the earth’s atmosphere and makes up about 1 %. Argon is continuously released into the air by decay of radioactive potassium-40. Pure form is obtained from fractional distillation of liquid air. Used in lighting products. It is often used in filling incandescent light bulbs. Some is mixed with krypton in fluorescent lamps. Crystals in the semiconductor industry are grown in argon atmospheres.
azeotrope → azeotropi
Azeotrope is a mixture of two liquids that boils at constant composition, i.e. the composition of the vapour is the same as that of the liquid. Azeotropes occur because of deviations in Raoult’s law leading to a maximum or minimum in the boiling point - composition diagram. The composition of an azeotrope depends on the pressure.
carbon → ugljik
Carbon has been known since ancient times. The origin of the name comes from the Latin word carbo meaning charcoal. Graphite form of carbon is a black, odourless, slippery solid. Graphite sublimes at 3825 °C. Diamond form is a clear or colored; an extremely hard solid. C60 is Buckminsterfullerine. Carbon black burns readily with oxidants. Carbon is made by burning organic compounds with insufficient oxygen. There are close to ten million known carbon compounds, many thousands of which are vital to organic and life processes. Radiocarbon dating uses the carbon-14 isotope to date old objects.
coal gas → ugljeni plin
Coal gas is a gas produced by the destructive distillation of coal, and contains approximately 50 % hydrogen, 35 % methane and 8 % carbon monoxide. The by-products of the production of coal gas are coal tar and coke.
coal tar → ugljeni katran
Coal tar is a material obtained from the destructive distillation of coal in the production of coal gas. The crude tar contains a large number of organic compounds (e.g. benzene, naphthalene, methylbenzene, etc.), which can be separated by fractional distillation.
detergent → deterdžent
Detergent is a substance added to water to improve its cleaning properties. Although water is a powerful solvent for many compounds, it will not dissolve grease and natural oils. Detergents are compounds that cause such nonpolar substances to go into solution in water. Soap is the original example, owing its action to the presence of ions formed from long-chain fatty acids ion (e.g. stearat ion, CH3(CH2)16COO-).
mineral oils → mineralna ulja
Mineral oils are oily liquids that are composed of hydrocarbons and are obtained as a product of petroleum, tar, coal, wood etc. distillation. They are used as lubricants.
Citing this page:
Generalic, Eni. "Destilacija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table