plastic → plastika
Plastic is a material that can be shaped by the application of heat or pressure. Most are based on synthetic polymers although some are the product of natural substances (such as cellulose derivatives, but excluding the rubbers.). They are usually light and permanent solids, being also heat and electric isolators. If the materials soften again when reheated, they are said to be thermoplastic. If, after fashioning, they resist further applications of heat, they are said to be thermoset.
polysaccharide → polisaharid
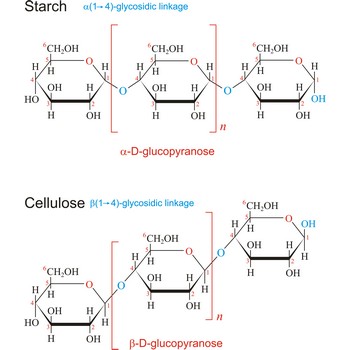
Polysaccharides are compounds consisting of a large number of simple sugars (monosaccharides) linked together by glycosidic bonds. When polysaccharides are composed of a single monosaccharide building block, they are termed homopolysaccharides. Heteropolysaccharides contain two or more different types of monosaccharide. Polysaccharides may have molecular weights of up to several million and are often highly branched. Since they have only the one free anomeric -OH group at the end of a very long chain, polysaccharides aren’t reducing sugars and don’t show noticeable mutarotation. The most common polysaccharides are cellulose, starch, and glycogen.
Chitin → Hitin
Chitin is a nitrogen-containing linear polysaccharide of ß(1->4) linked units of N-acetyl-ß-d-glucosamine. The structure of chitin is similar to cellulose except for the replacement hydroxyl group (-OH) at the carbon 2 with an acetyl amine group (–NH–CO–CH3). Chitin is the main component of the exoskeleton, or outer covering of insects, crustaceans, and arachnids. It is also found in the cell walls of certain fungi and algae. After cellulose, chitin is the second most abundant biopolymer in nature. It is insoluble in water, organic solvents, weak acids and lyes.
starch → škrob
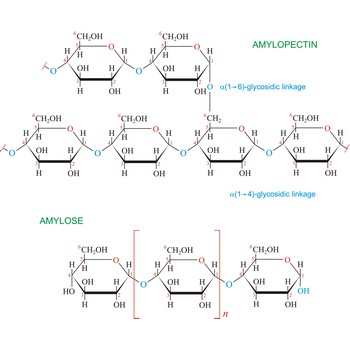
Starch (C6H10O5)x is a polysaccharide used by plants to stockpile glucose molecules. It is the major component of flour, potatoes, rice, beans, corn, and peas. Starch is a mixture of two different polysaccharides: amylose (about 20 %), which is insoluble in cold water, and amylopectin (about 80 %), which is soluble in cold water. Amylose is composed of unbranched chains of D-glucose units joined by α(1→4)-glycosidic linkages. Unlike amylose, which are linear polymers, amylopectin contains α(1→6)-glycoside branches approximately every 25 glucose units.
Starch digestion begins in the mouth via the action of amylase, a digestive enzyme present in saliva. The process is completed in the small intestine by the pancreatic amylase. The final products of starch digestion, glucose molecules, are absorbed into the intestinal bloodstream and transported to the liver. Like most enzymes, glycosidases are highly selective in their action. They hydrolyze only the α-glycoside links in starch and leave the β-glycoside links in cellulose untouched. Starch is important food stuff and is used in adhesives, and sizes, in laundering, pharmacy and medicine.
Citing this page:
Generalic, Eni. "Celuloza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table