lattice energy → energija kristalne rešetke
Lattice energy is the energy per ion pair required to separate completely the ions in a crystal lattice at a temperature of absolute zero.
molecular lattice → molekularna rešetka
Molecular lattice is a crystal lattice made molecules bonded together by intermolecular forces.
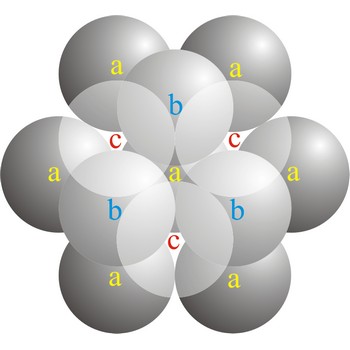
cubic close-packed structure → kubična gusta slagalina
In a cubic close-packed (ccp) arrangement of atoms, the unit cell consists of four layers of atoms. The top and bottom layers (a) contain six atoms at the corners of a hexagon and one atom at the center of each hexagon. The atoms in the second layer (b) fit into depressions in the first layer. The atoms in the third layer (c) occupy a different set of depressions than those in the first. The cubic close packed structure can be made by piling layers in the a-b-c-a-b-c-a-b-c... sequence.
cubic crystal system → kubični kristalni sustav
Cubic crystal system is also known as the isometric system. The Isometric crystal system characterizes itself by its three equivalent crystallographic axes perpendicular to each other.
a = b = c
α = β = γ = 90°
face-centered orthorhombic lattice → plošno centrirana ortorompska rešetka
Face-centered orthorhombic lattice (orthorhombic-F), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of each face of the unit cell. It has unit cell vectors a≠b≠c and interaxial angles α=β=γ=90°.
hexagonal lattice → heksagonska rešetka
Hexagonal lattice has lattice points at the twelve corners of the hexagonal prism and at the centers of the two hexagonal faces of the unit cell. It has unit cell vectors a=b≠c and interaxial angles α=β=90° and γ=120°.
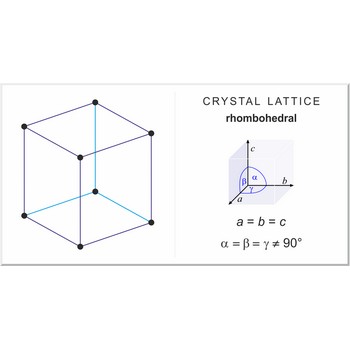
rhombohedral lattice → romboedarska rešetka
Rhombohedral (or trigonal) lattice has one lattice point at the each corner of the unit cell. It has unit cell vectors a=b=c and interaxial angles α=β=γ≠90°.
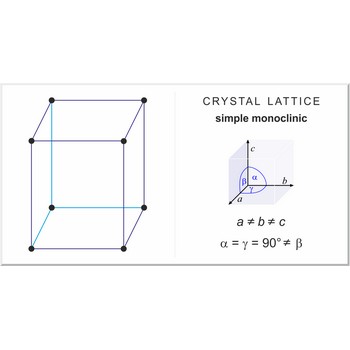
simple monoclinic lattice → jednostavna monoklinska rešetka
Simple or primitive monoclinic lattice (monoclinic-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a≠b≠c and interaxial angles α=γ=90°≠β.
simple orthorhombic lattice → jednostavna ortorompska rešetka
Simple or primitive orthorhombic lattice (orthorhombic-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a≠b≠c and interaxial angles α=β=γ=90°.
simple tetragonal lattice → jednostavna tetragonska rešetka
Simple or primitive tetragonal lattice (tetragonal-P) has one lattice point at the each corner of the unit cell. It has unit cell vectors a=b≠c and interaxial angles α=β=γ=90°.
Citing this page:
Generalic, Eni. "Body-centred cubic lattice." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table