gadolinium → gadolinij
Gadolinium was discovered by Jean de Marignac (France) in 1880. Named after the mineral gadolinite, named for J. Gadolin, a Finnish chemist and mineralogist. It is soft, ductile, silvery-white metal. Reacts slowly with water and oxygen. Dissolves in acids. Metal ignites and burns readily. Gadolinium is found with other rare earths in gadolinite and monazite sand. Used in steel alloying agents and the manufacture of electronic components.
germanium → germanij
Germanium was discovered by Clemens Winkler (Germany) in 1886. The origin of the name comes from the Latin word Germania meaning Germany. It is greyish-white semi-metal. Unaffected by alkalis and most (except nitric) acids. Stable in air and water. Germanium is obtained from refining copper, zinc and lead. Widely used in semiconductors. It is a good semiconductor when combined with tiny amounts of phosphorus, arsenic, gallium and antimony.
glass electrode → staklena elektroda
Glass electrode is a hydrogen-ion responsive electrode usually consisting of a bulb, or other suitable form, of special glass attached to a stem of high resistance glass complete with internal reference electrode and internal filling solution system. Glass electrode is also available for the measurement of sodium ions.
The glass electrode, which consists of a thin wall glass bulb, has an extremely high electrical resistance. The membrane of a typical glass electrode (with a thickness of 0.03 mm to 0.1 mm) has an electrical resistance of 30 MΩ to 600 MΩ. The surface of a glass membrane must be hydrated before it will function as a pH electrode. When a glass surface is immersed in an aqueous solution then a thin solvated layer (gel layer) is formed on the glass surface in which the glass structure is softer. This applies to both the outside and inside of the glass membrane.
The simplest explanation for the working of the thin glass electrode is that the glass acts as a weak acid (Glass-H).
The hydrogen ion activity of the internal solution is held constant. When a solution of different pH from the inside comes in contact with the outside of the glass membrane, the glass is either deprotonated or protonated relative to the inside of the glass. The difference in pH between solutions inside and outside the thin glass membrane creates electromotive force in proportion to this difference in pH.
weak electrolyte → slabi elektrolit
Weak electrolytes are those electrolytes which in water solutions dissociate only partially, giving ions and which are in equilibrium with undissociated molecules. Their water solutions conduct electric current weakly. For example, acetic acid partially dissociates into acetate ions and hydrogen ions, so that an acetic acid solution contains both molecules and ions.
Zimmermann-Reinhardt’s reagent → Zimmermann-Reinhardtov reagens
Zimmermann-Reinhardt’s reagent is a mixture of manganese(II) sulphate, sulphuric acid and phosphorus acid. It is used for preventing oxidation of chloride ion while titrating iron(II) ion with permanganate solution.
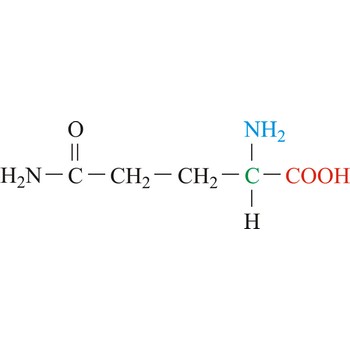
glutamine → glutamin
Glutamine is neutral amino acids with polar side chains. It serves as an important carrier of ammonia and contributes it to the formation of urea and purines. Glutamine is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders. It is synthesized by the enzyme glutamine synthetase from glutamate and ammonia.
- Abbreviations: Gln, Q
- IUPAC name: 2,5-diamino-5-oxopentanoic acid
- Molecular formula: C5H10N2O3
- Molecular weight: 146.14 g/mol
glycine → glicin
Glycine is the smallest amino acid and is unique because it lacks a side chain. This gives it more conformational freedom than any other amino acid. Glycine is often found in turns and loops where other amino acids would be sterically unacceptable. Although it is formally nonpolar, it’s very small side chain makes no real contribution to hydrophobic interactions. Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine.
- Abbreviations: Gly, G
- IUPAC name: 2-aminoacetic acid
- Molecular formula: C2H5NO2
- Molecular weight: 75.07 g/mol
gold → zlato
Gold has been known since ancient times. The origin of the name comes from the Latin word aurum meaning gold. It is soft, malleable, bright yellow metal. Unaffected by air, water, alkalis and most acids. Gold is found in veins in the crust, with copper ore and native. Used in electronics, jewellery and coins. It is a good reflector of infrared radiation, so a thin film of gold is applied to the glass of skyscrapers to reduce internal heating from sunlight.
haematite → hematit
Haematite is a mineral of iron(III) oxide Fe2O3. It is the most important ore of iron and usually occurs in two main forms: as a massive red kidney-shaped ore and as grey to black metallic crystals known as specular iron ore. Haematite is the major red colouring agent in rocks; the largest deposits are of sedimentary origin. In industry haematite is also used as a polishing agent (jeweller’s rouge) and in paints.
hafnium → hafnij
Hafnium was discovered by Dirk Coster (Denmark) and Georg Karl von Hevesy (Hungary) in 1923. The origin of the name comes from the Latin name Hafnia meaning Copenhagen. It is silvery, ductile metal. Exposed surfaces form oxide film. Resists alkalis and acids (except HF). Toxic. Metal ignites and burns readily. Hafnium is obtained from mineral zircon or baddeleyite. Used in reactor control rods because of its ability to absorb neutrons.
Citing this page:
Generalic, Eni. "Asparaginska kiselina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table