Zeeman, Pieter → Zeeman, Pieter
Pieter Zeeman (1865-1943) was a Dutch physicist who discovered the splitting of the spectral lines of a substance when placed in a magnetic field (known as the Zeeman effect). In 1902, Zeeman and Lorentz were jointly awarded the Nobel Prize in Physics, for their, extraordinary service they rendered by their researches into the influence of magnetism upon radiation phenomenon.
absorbance → apsorbancija
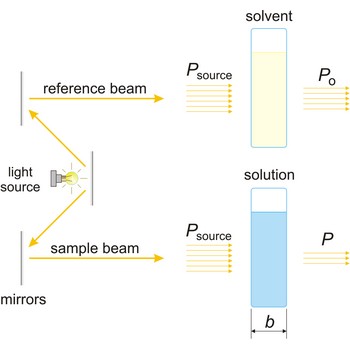
Absorbance (A) is a logarithm of the ratio of incident radiant power (Po) to transmitted radiant power (P) through a sample (excluding the effects on cell walls).
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
activity → aktivitet
Activity (a) is a thermodynamic function used in place of concentration in equilibrium constants for reactions involving nonideal gases and solutions. For the species i activity is defined as
where ai is the activity of the species i, ci is its molar concentration, and fi is a dimensionless quantity called the activity coefficient.
arsenic → arsen
Arsenic was discovered by Albertus Magnus (Germany) in 1250. The origin of the name comes from the Greek word arsenikon meaning yellow orpiment. It is steel-grey, brittle semi-metal. Resists water, acids and alkalis. Tarnishes in air, burns in oxygen. Highly toxic by inhalation or ingestion. Arsenic is found in mispickel (arsenopyrite). Many of its compounds are deadly poison and used as weed killer and rat poison. Used in semiconductors. Some compounds, called arsenides, are used in the manufacture of paints, wallpapers and ceramics.
Brownian motion → Braunovo gibanje
Brownian motion is the continuous random movement of small particles suspended in a fluid, which arise from collisions with the fluid molecules. First observed by the British botanist R. Brown (1773-1858) when studying pollen particles. The effect is also visible in particles of smoke suspended in a gas.
bismuth → bizmut
Bismuth was discovered by Claude Geoffroy (France) in 1753. The origin of the name comes from the German words Weisse Masse meaning white mass; now spelled wismut and bisemutum. It is hard, brittle, steel-grey metal with a pink tint. Stable in oxygen and water. Dissolves in concentrated nitric acid. Bismuth can be found free in nature and in minerals like bismuthine (Bi2S3) and in bismuth ochre (Bi2O3) Main use is in pharmaceuticals and low melting point alloys used as fuses.
colloid → koloid
Colloids are systems in which there are two or more phases, with one (the dispersed phase) distributed in the other (the continuous phase). Moreover, at least one of the phases has small dimensions, in the range between 1 nm and 1 μm (10-9 m – 10-6 m). Dimension, rather than the nature of the material, is characteristic. In this size range, the surface area of the particle is large with respect to its volume so that unusual phenomena occur, e.g., the particles do not settle out of the suspension by gravity and are small enough to pass through filter membranes. Macromolecules (proteins and other high polymers) are at the lower limit of this range; the upper limit is usually taken to be the point at which the particles can be resolved in an optical microscope.
Colloidal particles may be gaseous, liquid, or solid, and occur in various types of suspensions:
Sols - dispersions of small solid particles in a liquid.
Emulsions - colloidal systems in which the dispersed and continuous phases are both liquids.
Gels - colloids in which both dispersed and continuous phases have a three-dimensional network throughout the material.
Aerosols - colloidal dispersions of liquid or solid particles in a gas.
Foams - dispersions of gases in liquids or solids.
diffraction → difrakcija
Diffraction is the ability of a wave to bend around the edges of obstacles or holes. The effect is most noticeable when the obstacle or hole is comparable to the size of the wavelength
photoelectric threshold → fotoelektrični prag
Photoelectric threshold is a maximum length of electromagnetic wave which can still cause the photoelectric effect.
superconductivity → supravodljivost
Superconductivity is the phenomenon in which certain metals, alloys, and compounds below a certain temperature, the transition point (Tc), lose electrical resistance and magnetic permeability, i.e. have infinite electrical conductivity (Meissner effect and Josephson effect).
Citing this page:
Generalic, Eni. "Zeemanov efekt." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table