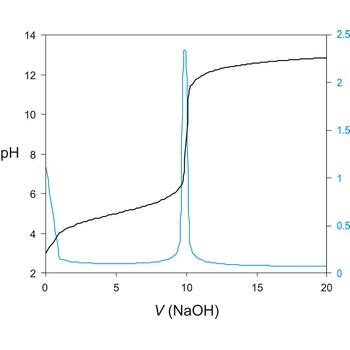
potentiometric titration → potenciometrijska titracija
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
qualitative analysis → kvalitativna analiza
Qualitative analysis involves determining the nature of a pure unknown compound or the compounds present in a mixture. Qualitative inorganic analysis is used to separate and detect cations and anions in a sample substance. According to their properties, cations are usually classified into six groups. Each group has a common reagent which can be used to separate them from the solution.
salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
significant figures → značajne znamenke
Measurements are not infinitely accurate: we must estimate measurement uncertainty. The number of significant figures is all of the certain digits plus the first uncertain digit.
Rules for significant figures:
- Disregard all initial zeros.
- Disregard all final zeros unless they follow a decimal point.
- All remaining digits including zeros between nonzero digits are significant.
| 0.0023 | has two significant figures |
| 0.109 | has three significant figures |
| 2.00 | has three significant figures |
| 70 | has one significant figure |
In addition and subtraction, the number of significant figures in the answer depends on the original number in the calculation that has the fewest digits to the right of the decimal point.
In multiplication and division, the number of significant figures in a calculated result is determined by the original measurement that has the fewest number of significant digits.
In a logarithm of a number, keep as many digits to the right of the decimal point as there are significant figures in the original number.
In an antilogarithm of a number, keep as many digits as there are digits to the right of the decimal point in the original number.
standard → standard
Standards are materials containing a known concentration of an analyte. They provide a reference to determine unknown concentrations or to calibrate analytical instruments.
The accuracy of an analytical measurement is how close a result comes to the true value. Determining the accuracy of a measurement usually requires calibration of the analytical method with a known standard. This is often done with standards of several concentrations to make a calibration or working curve.
A primary standard is a reagent that is extremely pure, stable, has no waters of hydration, and has a high molecular weight.
A secondary standard is a standard that is prepared in the laboratory for a specific analysis. It is usually standardised against a primary standard.
supercritical fluid chromatography → superkritična fluidna kromatografija
Supercritical fluid chromatography (SFC) is a hybrid of gas and liquid chromatography. SFC is of importance because it permits the separation and determination of a group of compounds that are not conveniently handled by either gas or liquid chromatography. These compounds are either nonvolatile or thermally labile so that gas chromatography cannot be used and they do not contain functional groups that make possible detection by liquid chromatography. SFC has been applied to a wide variety of materials including natural prodcuts, drugs, foods, pesticides and herbicides, fossil fuels, explosives and propellants.
Winkler’s method → Winklerova metoda
Winkler’s method was once a common method used to determine the dissolved oxygen concentration by titration. Now rarely used due to the accuracy and low price of oxygen meters.
The water sample is first treated with excess manganese(II) sulfate solution and then with an alkaline solution of potassium iodide. The Mn(OH)2 initially formed reacts with the dissolved oxygen. The amount of MnO(OH)2 formed is determined by reaction with iodide ion in acidic solution. The iodine formed may be titrated against standard thiosulfate solution, using starch as an indicator.
Citing this page:
Generalic, Eni. "Schrotterova aparatura za određivanje CO2." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table