magnesium → magnezij
Magnesium was discovered by Sir Humphry Davy (England) in 1808. The origin of the name comes from the Greek word Magnesia, a district of Thessaly. It is lightweight, malleable, silvery-white metal. Burns in air with a brilliant white flame and reacts with water as temperature elevates. Can ignite in air. React violently with oxidants. Magnesium is found in large deposits in the form of magnesite, dolomite and other minerals. It is usually obtained by electrolysis of melted magnesium chloride (MgCl2) derived from brines, wells and sea water. Used in alloys to make airplanes, missiles and other uses for light metals. Have structural properties similar to aluminium.
optical activity → optička aktivnost
Optical activity is the ability of a chiral molecule to rotate the plane of plane-polairzed light. Molecules of an optically active substance cannot be superimposed on their own mirror images, just as your left hand cannot be superimposed on your right when both are held palm-down.
thermal resistance → toplinski otpor
Heat always flows from a higher to a lower temperature level. The driving force for the heat flux lies in the temperature difference ΔT between two temperature levels. Analogous to Ohm’s law, the following holds:
where H = dQ/dt is heat flux, measured in watts, ΔT is temperature difference across the thermal resistance, measured in kelvin, and Rth is thermal resistance, measured in K/W.
For example, suppose there were two houses with walls of equal thickness; one is made of glass and the other of asbestos. On a cold day, heat would pass through the glass house much faster. The thermal restistance of asbestos is then higher than of glass.
If the thermal Ohm’s law is divided by the heat capacity C, Newton’s law of cooling is obtained:
where dT/dt is rate of cooling or heating, measured in K s-1, and C is heat capacity, measured in J K-1.
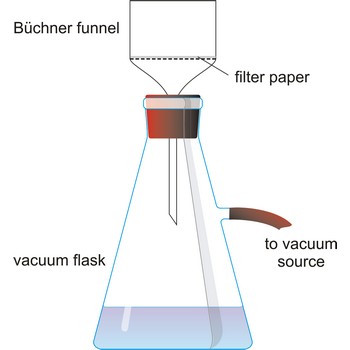
vacuum filtration → vakuum filtracija
Vacuum filtration is a technique for separating a solid product from a liquid. The mixture of solid and liquid is poured through a filter paper in a Buchner funnel. The solid is trapped by the filter and the liquid is drawn through the funnel into the flask below, by a vacuum.
Schrotter apparatus for determination of CO2 → Schrotterova aparatura za određivanje CO2
Schrötter decomposition apparatus (Schrötter's alkalimeter) is used to determining the carbonate content in samples of limestone, gypsum, dolomite, or baking powder by loss of weight. The apparatus is named after the Austrian chemist Anton Schrötter von Kristelli (1802-1875), who devised it in 1871. The size of the filled apparatus (apparatus is 16 cm high) is such that it weights less than 75 g, and can be placed on the pan of an analytical balance.
Procedure: Weigh about 0.5 g of the powdered carbonate sample and introduce it into the decomposition flask C. Pour into the drying tube A 2-3 mL of concentrated sulphuric acid (H2SO4), and to the dropping funnel B add about 10-15 mL of hydrochloric acid (w(HCl) = 15 %). Weigh the whole apparatus. Open the upper taps of both parts and allow the hydrochloric acid from B to run slowly down on to the powdered sample. The evolved CO2 escapes through the strong sulphuric acid and is thus thoroughly dried. When further addition of acid produces no more evolution of CO2, warm the apparatus up to 80 °C so as to expel the CO2 from the solution. Connect the upper tap of the drying tube A to a water pump and draw a slow current of air through the apparatus until completely cool. Open the upper taps for a moment to equalize the internal and external pressure and weight the apparatus again. The weight loss is equal to the weight of carbon dioxide liberated from the carbonates.
Citing this page:
Generalic, Eni. "Hirschov lijevak." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table