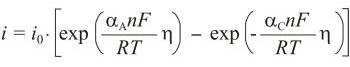blank determination → slijepa proba
Blank determination is a procedure of determining which follows all steps of analysis but in the absence of a sample. It is used for detection and compensation of systematic analysis mistakes.
blast furnace → visoka peć
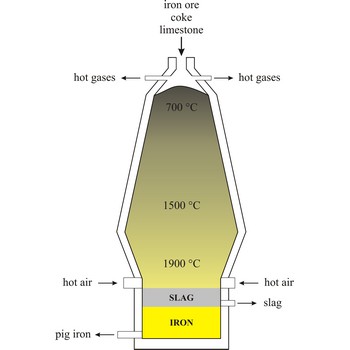
Blast furnace is a furnace for smelting of iron from iron oxide ores (hematite, Fe2O3 or magnetite, Fe3O4). Coke, limestone and iron ore are poured in the top, which would normally burn only on the surface. The hot air blast to the furnace burns the coke and maintains the very high temperatures that are needed to reduce the ore to iron. The reaction between air and the fuel generates carbon monoxide. This gas reduces the iron(III) oxide in the ore to iron.
Because the furnace temperature is in the region of 1500 °C, the metal is produced in a molten state and this runs down to the base of the furnace.
The production of iron in a blast furnace is a continuous process. The furnace is heated constantly and is re-charged with raw materials from the top while it is being tapped from the bottom. Iron making in the furnace usually continues for about ten years before the furnace linings have to be renewed.
bomb calorimeter → kalorimetrijska bomba
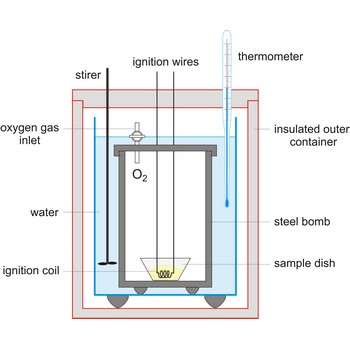
Bomb calorimeter is a type of constant-volume calorimeter used in measuring the heat of combustion of samples which can be burned in oxygen. Four essential parts are required in any bomb calorimeter:
- a bomb or vessel in which the combustible charges can be burned,
- a bucket or container for holding the bomb in a measured quantity of water, together with a stirring mechanism,
- an insulating jacket to protect the bucket from transient thermal stresses during the combustion process, and
- a thermometer or other sensor for measuring temperature changes within the bucket.
calcification → kalcifikacija
Calcification is a process of deposition of calcium salts in body tissues.
calcination → kalciniranje
Calcination is a process of driving out crystal water from crystal salts by heating.
cementation → cementacija
Cementation is any metallurgical process in which the surfaces of a metal is impregnated by some other substance, especially an obsolete process for making steel by heating bars of wrought iron to red heat for several days in a bed of charcoal.
chemical → kemikalija
Chemicals are a common name for all chemical products or substances prepared by means of chemical-technologic processes.
Born-Haber cycle → Born-Haberov kružni proces
Born-Haber cycle is a cycle of reactions used for calculating the lattice energies of ionic crystalline solids. For a compound MX, the lattice energy is the enthalpy of the reaction
The standard enthalpy of formation of the ionic solid is the enthalpy of the reaction
The cycle involves equating this enthalpy (which can be measured) to the sum of the enthalpies of a number of steps proceeding from the elements to the ionic solid. The steps are:
1) Atomization of the metal
2) Atomization of the nonmetal
3) Ionisation of the metal
This is obtained from the ionisation potential.
4) Ionisation of the nonmetal
This is electron affinity.
5) Formation of the ionic solids
Equation of the enthalpies gives
from which ΔHL can be found.
Butler-Volmer equation → Butler-Volmerova jednadžba
Butler-Volmer equation is an activation controlled reaction, the one for which the rate of reaction is controlled solely by the rate of the electrochemical charge transfer process, which is in turn an activation-controlled process. This gives rise to kinetics that are described by the Butler-Volmer equation:
where io is exchange current density, η is overpotential (η = E - Eo), n is number of electrons, αA is anodic transfer coefficient, and αC is cathodic transfer coefficient
chemical corrosion → kemijska korozija
Chemical corrosion is a damaging process of some materials with a chemical agent.
Citing this page:
Generalic, Eni. "Carnotov kružni proces." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table