percentage composition of atom in the molecule → postotni sastav atoma u molekuli
Percentage composition of atom in the molecule is a structure of compound presented in the shape of a percentage of its mass, which comes from every element.
solution composition → sastav otopine
Solutions are homogenous mixtures of several components. The component which is found in a greater quantity is called the solvent and the other components are called solutes. Quantitative composition of a solution can be expressed by concentration (amount, mass, volume and number), by fraction (amount, mass, and volume), ratio (amount, mass, and volume) and by molality. Amount, mass, and volume ratio are numerical, nondimensional units and are frequently expressed as percentage (% = 1/100), promile (‰ = 1/1000) or parts per million (ppm = 1/1 000 000). If it is not defined, it is always related to the mass ratio.
thermodynamic laws → termodinamički zakoni
Thermodynamic laws are the foundation of the science of thermodynamics:
First law: The internal energy of an isolated system is constant; if energy is supplied to the system in the form of heat dq and work dw, then the change in energy dU = dq + dw.
Second law: No process is possible in which the only result is the transfer of heat from a reservoir and its complete conversion to work.
Third law: The entropy of a perfect crystal approaches zero as the thermodynamic temperature approaches zero.
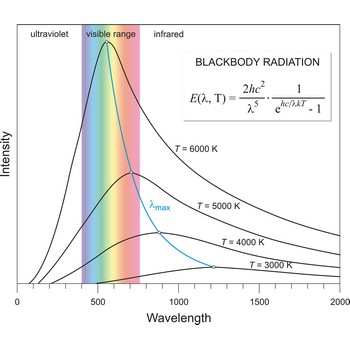
blackbody radiation → zračenje crnog tijela
Blackbody radiation is the radiation emitted by a perfect blackbody, i.e., a body which absorbs all radiation incident on it and reflects none. The primary law governing blackbody radiation is the Planck Radiation Law, which governs the intensity of radiation emitted by unit surface area into a fixed direction (solid angle) from the blackbody as a function of wavelength for a fixed temperature. The Planck Law can be expressed through the following equation
where λ is the wavelength, h is Planck’s constant, c is the speed of light, k is the Boltzmann constant, and T is the temperature.
polymorphic transition → polimorfni prijelaz
Polymorphic transition is a reversible transition of a solid crystalline phase at a certain temperature and pressure to another phase of the same chemical composition with a different crystal structure. For examples, the transitions of quartz (SiO2) at 1 143 K to tridymite, and at 1 743 K to cristobalite.
ideal gas law → jednadžba stanja idealnog plina
The generalized ideal gas law is derived from a combination of the laws of Boyle and Charles. Ideal gas law is the equation of state
which defines an ideal gas, where p is pressure, V molar volume, T temperature, and R the molar gas constant (8.314 JK-1mol-1).
visible radiation → vidljivo zračenje
Human eye can only see electromagnetic radiation of wavelengths form 400 nm to 760 nm. This narrow part of electromagnetic spectrum is called visible radiation. Visible (white) light is a mixture of light of all kind of colours, it can be separated, with the help of a glass prism, into its component colours - visible light spectrum, and each colour corresponds to a certain area of wavelengths:
| Colour | Wavelength / nm |
|---|---|
| purple | 400 - 450 |
| blue | 450 - 500 |
| green | 500 - 570 |
| yellow | 570 - 590 |
| orange | 590 - 620 |
| red | 620 - 760 |
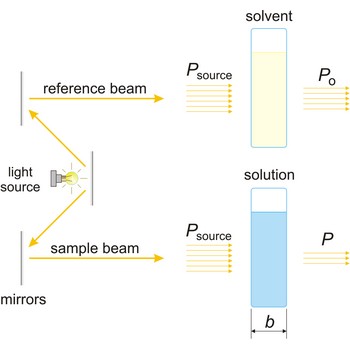
absorbance → apsorbancija
Absorbance (A) is a logarithm of the ratio of incident radiant power (Po) to transmitted radiant power (P) through a sample (excluding the effects on cell walls).
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
abundance of elements → rasprostranjenost elemenata
Elements in nature are mostly found in different compounds and, rarely, in the free (elementary) state. In Earth’s crust the most abundant of all elements is oxygen (with 49.5 %), then silicon (25 %), aluminium (7.5 %), iron (4.7 %), calcium (3.4 %), sodium (2.6 %), potassium (2.4 %), magnesium (1.9 %) and hydrogen (1.9 %). These nine elements make up almost 99 % of the Earth’s composition.
abundance of substances → rasprostranjenost tvari
Abundance of substances is the ratio of the total mass of a specified element in the Earth’s crust to the total mass of the Earth’s crust. It is often expressed as a percentage.
Citing this page:
Generalic, Eni. "Zakon o određenom sastavu." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table