enzyme → enzim
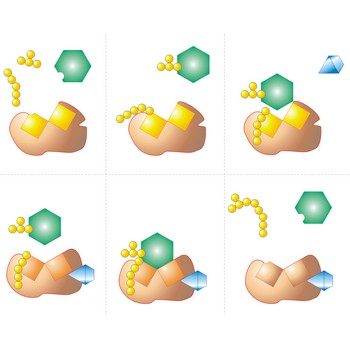
Enzyme is a protein that acts as a catalyst in biochemical reactions. Each enzyme is specific to a particular reaction or a group of similar reactions. Many require the association of certain nonprotein cofactors in order to function. The molecule undergoing a reaction (the substrate) binds to a specific active site on the enzyme molecule to form a short-lived intermediate: this greatly increases (by a factor of up to 1020) the rate at which the reaction proceeds to form the product.
micelle → micele
Micelle is an electrically charged colloidal particle, usually organic in nature, composed of aggregates of large molecules, e.g., in soaps and surfactants. For aqueous solutions, the hydrophilic end of the molecule is on the surface of the micelle, while the hydrophobic end (often a hydrocarbon chain) points toward the centre.
natural gas → prirodni plin
Natural gas is a naturally occurring mixture of gaseous hydrocarbons. The approximate composition of natural gas is 85 % methane, 10 % ethane, 3 % propane, with lesser amounts of butane, and other higher alkanes. Natural gas is used as a fuel and for the manufacture of chemicals.
electron → elektron
The electron is an elementary particle with a negative electric charge of (1.602 189 2±0.000 004 6)×10-19 C and a mass of 1/1837 that of a proton, equivalent to (9.109 534±0.000 047)×10-31 kg.
In 1897 the British physicist Joseph John (J.J.) Thomson (1856-1940) discovered the electron in a series of experiments designed to study the nature of electric discharge in a high-vacuum cathode-ray tube. Thomson interpreted the deflection of the rays by electrically charged plates and magnets as evidence of bodies much smaller than atoms that he calculated as having a very large value for the charge to mass ratio. Later he estimated the value of the charge itself.
Electrons are arranged in from one to seven shells around the nucleus; the maximum number of electrons in each shell is strictly limited by the laws of physics (2n2). The outer shells are not always filled: sodium has two electrons in the first shell (2×12 = 2), eight in the second (2×22 = 8), and only one in the third (2×32 = 18). A single electron in the outer shell may be attracted into an incomplete shell of another element, leaving the original atom with a net positive charge. Valence electrons are those that can be captured by or shared with another atom.
Electrons can be removed from the atoms by heat, light, electric energy, or bombardment with high-energy particles. Decaying radioactive nuclei spontaneously emit free electrons, called β particles.
equal-arm balance → vaga s jednakim krakovima
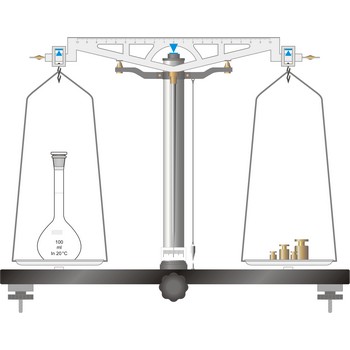
The simplest type of balance, the equal-arm balance, is an application of a first class lever. The beam of the balance is supported on a central knife-edge, usually of agate, which rests upon a plane agate plate. The point of support is called the fulcrum. Two pans of equal weight are suspended from the beam, one at each end, at points equidistant from the fulcrum. A long pointer attached at right angles to the beam at the fulcrum indicates zero on a scale when the beam is at rest parallel to a level surface.
To prevent the knife-edge from becoming dull under the weight of the beam and pans the balance is equipped with a special device called an arrest. The arrest is operated by means of milled knob underneath the base plate in the middle and in front of the balance (sometimes the arrest knob is at one side of the balance).
The object to be weighed is placed on one pan, and standard weights are added to the other until the balance of the beam is established again. When not in use and during loading or unloading of the pans, the balance should be arrested.
equilibrium constant → konstanta ravnoteže
The equilibrium constant (K) was originally introduced in 1863 by Norwegian chemists C.M. Guldberg and P. Waage using the law of mass action. For a reversible chemical reaction represented by the equation
chemical equilibrium occurs when the rate of the forward reaction equals the rate of the back reaction, so that the concentrations of products and reactants reach steady-state values.
The equilibrium constant is the ratio of chemical activities of the species A, B, C, and D at equilibrium.
To a certain approximation, the activities can be replaced by concentrations.
For gas reactions, partial pressures are used rather than concentrations
The units of Kp and Kc depend on the numbers of molecules appearing in the stoichiometric equation (a, b, c, and d).
The value equilibrium constant depends on the temperature. If the forward reaction is exothermic, the equilibrium constant decreases as the temperature rises. The equilibrium constant shows the position of equilibrium. A low value of K indicates that [C] and [D] are small compared to [A] and [B]; i.e. that the back reaction predominates.
The equilibrium constant is related to ΔrG°, the standard Gibbs free energy change in the reaction, by
oligomer → oligomer
Oligomer is a substance consisting of molecules of intermediate relative molecular mass (molecular weight), the structure of which essentially comprises the multiple repetitions of units derived, actually or conceptually, from molecules of low relative molecular mass. In contrast to a polymer, the properties of an oligomer can vary significantly with the removal of one or a few of its units.
oligosaccharide → oligosaharid
Oligosaccharides are carbohydrates containing from three to ten monosaccharide units, each joined to the next by a glycoside bond.
Citing this page:
Generalic, Eni. "Zakon o određenom sastavu." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table