binary solution → binarna otopina
Binary solution is a mixture of two liquids that are completely miscible one with another. The boiling point of binary solution depends upon the solution composition and there can be three cases:
1. the boiling points of solutions of all compositions lie between the boiling points of clean liquids
2. the boiling points of solutions of any composition lie above the boiling points of clean liquids
3. the boiling points of solutions of some compositions lie below the boiling points of clean liquids
Celsius temperature scale → Celsiusova temperaturna skala
For value of zero in Celsius temperature scale the freezing point of water at a pressure of 101 325 Pa is taken. The boiling point of water at a pressure of 101 325 Pa is taken as another reference point. This range is divided into 100 equal parts, and each part is an equivalent to 1 °C. Units of Celsius temperature scale (°C) and thermodynamic temperature scale (K) are identical
1 °C = 1 K.
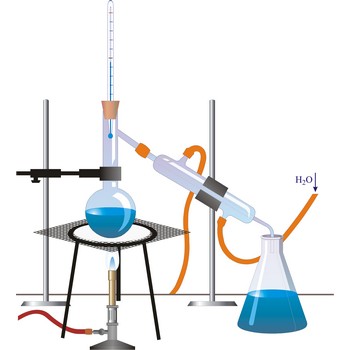
distillation → destilacija
Distillation is a process of boiling a liquid and condensing and collecting the vapour. The liquid collected is the distillate. The usual purpose of distillation is purification or separation of the components of a mixture. This is possible because the composition of the vapour is usually different from that of liquid mixture from which it is obtained. Petrol, kerosene, fuel oil, and lubricating oil are produced from petroleum by distillation.
ebullioscopic constant → ebulioskopska konstanta
Ebullioscopic constant (Eb) is the constant that expresses the amount by which the boiling point Tb of a solvent is raised by a nondissociating solute, through the relation
where b is the molality of the solute.
evaporation → isparavanje
Evaporation is the change of state of a liquid into a vapour at a temperature below the boiling point of the liquid.
heat of vaporisation → toplina isparavanja
Heat of vaporisation or enthalpy of vaporisation is the heat required to convert a substance from the liquid to the gaseous state with no temperature change (also called latent heat of vaporization).
latent heat → latentna toplina
Latent heat (L) is the quantity of heat absorbed or released when a substance changes its physical phase at constant temperature (e.g. from solid to liquid at the melting point or from liquid to gas at the boiling point).
low-weight fractions → lake frakcije
Low-weight (petroleum) fractions have low boiling points and short carbohydrates chains.
Citing this page:
Generalic, Eni. "Vrelište." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table