ionic strength → ionska jakost otopine
Ionic strength (μ or I) is a measure of the total concentration of ions in a solution, defined by
where zi is the charge of ionic species i and ci is its concentration.
lithium → litij
Lithium was discovered by Johan August Arfvedson (Sweden) in 1817. The origin of the name comes from the Greek word lithos meaning stone, apparently because it was discovered from a mineral source whereas the other two elements, sodium and potassium, were discovered from plant sources. It is soft silvery-white metal. Lightest of metals. Reacts slowly with water and oxygen. Flammable. Can ignite in air. Reacts with water to give off a flammable gas. Lithium is obtained by passing electric charge through melted lithium chloride and from the silicate mineral called spodumene [LiAl(Si2O6)]. Used in batteries. Also for certain kinds of glass and ceramics. Some is used in lubricants.
volumetry → volumetrija
Volumetry consists of adding an equivalent amount of a reagent of exactly known concentration to the analyte. From stechiometrical proportion and added volume of reagent the quantity of matter in question can be calculated.
zero-order reaction → reakcija nultog reda
Zero-order reaction is a reaction for which the rate of reaction is independent of the concentration of reactants.
mutarotation → mutarotacija
Mutarotation is the change in optical rotation accompanying epimerization. In carbohydrate chemistry this term usually refers to epimerization at the hemiacetal carbon atom. In general α- and β-form are stable solids, but in solution they rapidly equilibrate. For example, D-glucose exists in an equilibrium mixture of 36 % α-D-glucopyranose and 64 % β-D-glucopyranose, with only a tiny fraction in the open-chain form. The equilibration occurs via the ring opening of the cyclic sugar at the anomeric center with the acyclic form as the intermediate. Mutarotation was discovered by French chemist Augustin-Pierre Dubrunfaut (1797-1881) in 1846.
Nernst’s division law → Nernstov zakon razdjeljenja
Nernst’s division law states that a substance is divided between two solvents in a way that proportion of concentrations of that substance is at certain temperatures constant, under the condition that both solvents are in the same molecular state. Division coefficient is a proportion of substance concentration in solvents A i B at a defined temperature.
Appearance of division is used for substance extraction.
osmotic pressure → osmotski tlak
Osmotic pressure (Π) is the excess pressure necessary to maintain osmotic equilibrium between a solution and a pure solvent separated by a membrane permeable only to the solvent. In an ideal dilute solution
where cB is the amount-of-substance concentration of the solute, R is the molar gas constant, and T the temperature.
Ostwald’s dilution law → Ostwaldov zakon razrjeđenja
Ostwald’s dilution law is a relation for the concentration dependence of the molar conductivity Λ of an electrolyte solution, viz.
where c is the solute concentration, Kc is the equilibrium constant for dissociation of the solute, and L0 is the conductivity at cΛ = 0. The law was first put forward by the German chemist Wilhelm Ostwald (1853-1932).
pH → pH
pH is a convenient measure of the acid-base character of a solution, usually defined by
where c(H+) is the concentration of hydrogen ions in moles per litre. The more precise definition is in terms of activity rather than concentration.
A solution of pH 0 to 7 is acid, pH of 7 is neutral, pH over 7 to 14 is alkaline.
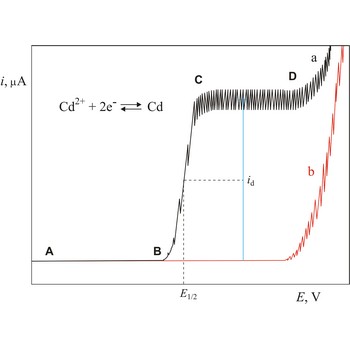
polarogram → polarogram
Polarogram is a graph of current versus potential in a polarographic analysis. The position of a polarographic wave in a polarogram along the x axis (E1/2) provides an identity of the substance while the magnitude of the limiting diffusion current (id) provides the concentration of this substance.
Citing this page:
Generalic, Eni. "Volumenska koncentracija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table