solubility → topljivost
Solubility is the maximum amount of solute that dissolves in a given quantity of solvent at a specific temperature. Generally, for a solid in a liquid, solubility increases with temperature; for a gas, solubility decreases. Common measures of solubility include the mass of solute per unit mass of solution (mass fraction), mole fraction of solute, molality, molarity, and others.
solubility product constant → konstanta produkta topljivosti
Solubility product constant (Ksp) (or the solubility product) is the product of the molar concentrations of the constituent ions, each raised to the power of its stoichiometric coefficient in the equilibrium equation. For instance, if a compound AaBb is in equilibrium with its solution
the solubility product is given by
spectrophotometer → spektrofotometar
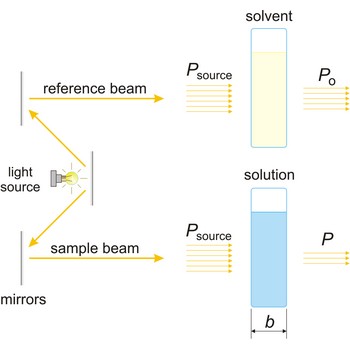
Spectrophotometer is an instrument for measuring the amount of light absorbed by a sample.
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
standard → standard
Standards are materials containing a known concentration of an analyte. They provide a reference to determine unknown concentrations or to calibrate analytical instruments.
The accuracy of an analytical measurement is how close a result comes to the true value. Determining the accuracy of a measurement usually requires calibration of the analytical method with a known standard. This is often done with standards of several concentrations to make a calibration or working curve.
A primary standard is a reagent that is extremely pure, stable, has no waters of hydration, and has a high molecular weight.
A secondary standard is a standard that is prepared in the laboratory for a specific analysis. It is usually standardised against a primary standard.
tear gas → suzavac
Tear gases is the common name for substances which, in low concentrations, cause pain in the eyes, flow of tears and difficulty in keeping the eyes open. Tear gases are used mainly in military exercises and in riot control, etc., but have also been used as a method of warfare. Irritating gases have been used in war since ancient times but it was not until after the Second World War that a more systematic search for effective substances was started. Among a long series of substances, three have become of greater importance than the others. These substances are chloroacetophenone (codename CN), orto-chlorobenzylidene-malononitrile (CS) and dibenz(b,f)-1,4-oxazepine (CR).
technetium → tehnecij
Technetium was discovered by Carlo Perrier and Emilio Segre (Italy) in 1937. The origin of the name comes from the Greek word technikos meaning artificial. It is silvery-grey metal. Resists oxidation but tarnishes in moist air and burns in high oxygen environment. First synthetically produced element. Radioactive. Technetium is made first by bombarding molybdenum with deuterons (heavy hydrogen) in a cyclotron. Added to iron in quantities as low as 55 part-per-million transforms the iron into a corrosion-resistant alloy.
thermometer → termometar
Thermometers are devices for measuring temperature. Linear and volume thermal expansion are macroscopic properties of matter, which can be easily measured, relative to measurements of microscopic properties, on the basis of which, temperature is defined. Thermometers based on thermal expansion are secondary instruments that is, they have to be calibrated in comparison to a standard thermometer. In a thermometer with liquid, mercury or alcohol is placed in a small glass container. If temperature increases, the liquid undergoes volume expansion and rises in a capillary. The level of the raised liquid is the measure of temperature. Mercury thermometers measure temperatures in the temperature range between -39 °C and 300 °C. Alcohol thermometers measure lower temperatures. Bimetal thermometers have a spiral spring, which consists of two metals with different coefficients of linear expansion. When temperature changes, metals undergo different change in length and the consequence twisting of the spring is transferred to a pointer, the deflection of which is the measure of temperature.
titar → titar
Titar (T) is a mass of titrated matter which is equivalent to 1 cm3 of solution. It is shown as T = 2.356 mg HCl / 1.0 cm3 NaOH, 0.1000 moldm-3, and it is usually shown in a table form. If the concentration of used standard solution (c) differs from one outlined in the table data (c0), the factor of correction (f) is induced
Titar is usually used in industrial operational laboratories where from titar tables mass or percentage of the ingredient in question is directly read.
water ion product → ionski produkt vode
Water ion product (Kw) is a concentration product of hydrogen and hydroxide ions. For the reaction:
the equilibrium expression would be:
Note that all pure liquid terms are omitted, hence H2O does not appear in the denominator. At 25 °C, Kw = 1.0×10-14 mol2dm-6 = (Ka)(Kb)
water hardness → tvrdoća vode
Hardness is defined as the concentrations of calcium and magnesium ions expressed in terms of calcium carbonate. These minerals in water can cause some everyday problems. They react with soap and produce a deposit called soap curd that remains on the skin and clothes and, because it is insoluble and sticky, cannot be removed by rinsing.
Hard water may also shorten the life of plumbing and water heaters. When water containing calcium carbonate is heated, a hard scale is formed that can plug pipes and coat heating elements. Scale is also a poor heat conductor. With increased deposits on the unit, heat is not transmitted to the water fast enough and overheating of the metal causes failure. Build-up of deposits will also reduce the efficiency of the heating unit, increasing the cost of fuel.
There are two types of water hardness, temporary and permanent.
Temporary Hardness is due to the bicarbonate ion, HCO3-, being present in the water. This type of hardness can be removed by boiling the water to expel the CO2, as indicated by the following equation:
Permanent hardness is due to calcium and magnesium nitrates, sulphates, and chlorides etc. This type of hardness cannot be eliminated by boiling.
| Water supply classification | |
|---|---|
| Hardness | Concentration of Calcium carbonate (mg/L) |
| Soft Water | 0 to 75 |
| Medium Hard Water | 75 to 150 |
| Hard Water | 150 to 300 |
| Very Hard Water | over 300 |
Citing this page:
Generalic, Eni. "Volumenska koncentracija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table