titrant → titrant
Titrant is the substance that quantitatively reacts with the analyte in a titration. The titrant is usually a standard solution added carefully to the analyte until the reaction is complete. The amount of analyte is calculated from the volume and concentration of titrant required for the complete reaction.
universal gas constant → univerzalna plinska konstanta
Universal gas constant R has the value of 8.314 472(15) J K-1 mol-1. It corresponds to the volume work performed by one mole of gas heated by 1 K at standard pressure.
galvanic celll → galvanski članak
Galvanic cell (voltaic cell) is a simple device with which chemical energy is converted into electrical energy. Galvanic cells consist of two separate compartments called half cells containing electrolyte solutions and electrodes that can be connected in a circuit. Two dissimilar metals (e.g., copper and zinc) are immersed in an electrolyte. If the metals are connected by an external circuit, one metal is reduced (i.e., gains electrons) while the other metal is oxidized (i.e., loses electrons).
In the example above, copper is reduced and zinc is oxidized. The difference in the oxidation potentials of the two metals provides the electric power of the cell.
A voltaic cell can be diagrammed using some simple symbols. In the diagram the electrodes are on the outer side of the diagram and a vertical line (|) is used to separate the electrode from the electrolyte solution found in the compartment. A double vertical line (||) is used to separate the cell compartments and is symbolic of the salt bridge. Usually in a diagram the species oxidized is written to the left of the double slash. Here is an example of the Daniell cell:
The names refer to the 18th-century Italian scientists Alessandro Volta (1745-1827) and Luigi Galvani (1737-1798).
gas liquefying → ukapljivanje plinova
In order to achieve transition of a gas into liquid state it is necessary to lower its temperature, or decrease its volume, or increase its pressure. Above the critical temperature it is impossible to liquefy a gas. When liquefying a gas by Linde’s procedure, dampening or Joule-Thomson’s effect is used. First, the compressed air from the compressor is cooled with cooling water, the cooled air expands at a lower pressure in the dampening valve at which it cooled. The cooled air now returns to the compressor, cooling down the expanding air. By repeating this process the air is cooled enough to transit to the liquid state.
gasoline → motorni benzin
Gasoline is a complex mixture of volatile hydrocarbons that may have between 5 to 12 carbons. The major components are branched-chain paraffins, cycloparaffins, and aromatics. Gasoline is most often produced by the fractional distillation of crude oil as the fraction of hydrocarbons in petroleum boiling between 30 °C and 200 °C. The quality of a fuel is measured with its octane number. Octane number is the measure of the resistance of gasoline against detonation or preignition of the fuel in the engine. The higher the octane number, the more compression the fuel can withstand before detonating. The octane number is determined by comparing the characteristics of a gasoline to isooctane with good knocking properties (octane number of 100) and heptane with bad (octane number of 0).
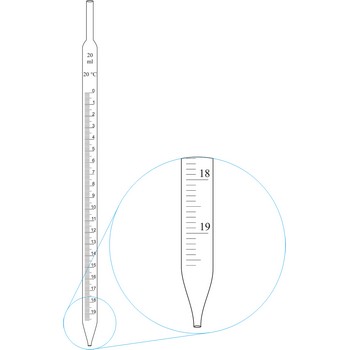
graduated pipette → graduirana pipeta
Graduated pipettes (Mohr pipette) have a scale divided into units of one and of 1/10th of a millilitre. Because of their wide necks it is less accurate than the volumetric pipette. They are used when taking volume of solutions in which accuracy does not have to be very high. By sucking in (with mouth, propipette or a water pump) the liquid is pulled in a little bit above the mark and the opening of the pipet is closed with a forefingertip. Outer wall of pipet is wiped and, with a slight forefinger loosening, the liquid is released until it reaches the mark 0. A pipette is emptied out by lifting the forefinger off and letting the liquid flow out of the pipette freely.
heat capacity → toplinski kapacitet
Heat capacity is defined in general as dQ/dT, where dQ is the amount of heat that must be added to a system to increase its temperature by a small amount dT. The heat capacity at a constant pressure is Cp = (∂H/∂T)p; that at a constant volume is CV = (∂E/∂T)V, where H is enthalpy, E is internal energy, p is pressure, V is volume, and T is temperature. An upper case C normally indicates the molar heat capacity, while a lower case c is used for the specific (per unit mass) heat capacity.
Hirsch funnel → Hirschov lijevak
Hirsch funnels are essentially smaller Büchner funnels and primarily used to collect a desired solid from a relatively small volume of liquid (1-10 mL). The main difference is that the plate is much smaller, while the walls of the funnel angle outward instead of being vertical. It is named after the German chemist Robert Hirsch (1856-1913).
weber → veber
Weber (Wb) is the SI derived unit of magnetic flux. The weber is the magnetic flux which, linking a circuit of one turn, produces in it an electromotive force of one volt as it is reduced to zero at a uniform rate in one second (Wb = V·s). The unit was named after the German scientist W.E. Weber (1804-1891).
Citing this page:
Generalic, Eni. "Volt." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table