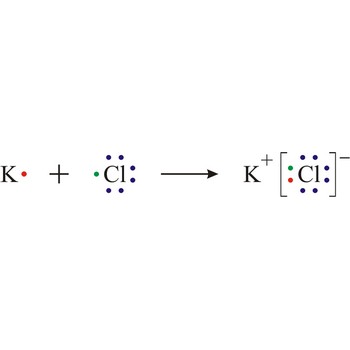vapour pressure → tlak pare
Vapour pressure is the pressure of a gas in equilibrium with a liquid (or, in some usage, a solid) at a specified temperature.
hydrophobic interaction → hidrofobne interakcije
Hydrophobic interaction is the tendency of hydrocarbons (or of lipophilic hydrocarbon-like groups in solutes) to form intermolecular aggregates in an aqueous medium, and analogous intramolecular interactions. The name arises from the attribution of the phenomenon to the apparent repulsion between water and hydrocarbons. Use of the misleading alternative term hydrophobic bond is discouraged.
hydrosphere → hidrosfera
Hydrosphere (from the Greek for water sphere) is a discontinuous layer of water on, under, and over the Earth's surface. It includes all liquid and frozen surface waters, groundwater held in soil and rock, and atmospheric water vapour. Water continuously circulates between these reservoirs in what is called the hydrologic cycle, which is driven by energy from the Sun.
| Reservoir | V / 106 km3 | w / % |
|---|---|---|
| oceans | 1 370.0 | 97.25 |
| ice caps and glaciers | 29.0 | 2.05 |
| groundwater | 9.5 | 0.68 |
| lakes, rivers | 0.127 | 0.01 |
| soil moisture | 0.065 | 0.005 |
| atmosphere (as liquid equivalent of water vapour) | 0.013 | 0.001 |
| biosphere | 0.0006 | 0.00004 |
| TOTAL | 1 408.7 | 100 |
ionic bond → ionska veza
Ionic bond is a strong force of attraction holding atoms together in a molecule or crystal. Typically chemical bonds have energies of about 100 kJ mol-1. Ionic bond is a bond at which one of the participants, during the procedure of bonding, gives away its unpaired electrons to another atom so that both can achieve electron arrangement of the closest noble gas. In order to form an ionic bond one of the atoms must cross to the positively charged ion by losing certain number of electrons and the other atom must receive those electrons and cross to the negatively charged ion.
vertical ionisation energy → vertikalna energija ionizacije
Vertical ionisation energy is the energy required to remove an electron from an atom, molecule, or ion in the gas phase without moving any nuclei. The vertical ionisation energy is greater than or equal to the adiabatic ionisation energy.
weak acid → slaba kiselina
Weak acid is an acid that incompletely dissociated in aqueous solution. Acetic acid is an example of a weak acid
weak electrolyte → slabi elektrolit
Weak electrolytes are those electrolytes which in water solutions dissociate only partially, giving ions and which are in equilibrium with undissociated molecules. Their water solutions conduct electric current weakly. For example, acetic acid partially dissociates into acetate ions and hydrogen ions, so that an acetic acid solution contains both molecules and ions.
iridium → iridij
Iridium was discovered by Smithson Tennant (England) in 1803. The origin of the name comes from the Latin word iris, meaning rainbow, because its salts are highly colored. It is heavy, brittle, white metal. Unreactive in air, water and acids. Attacked by fused NaOH. Metal ignites and burns readily. Iridium is found in gravel deposits with platinum. Used with osmium to tip gold pen points, to make crucible and special containers. Also to make alloys used for standard weights and measures and heat-resistant alloys. Also as hardening agent for platinum.
Joule-Thomson coefficient → Joule-Thompsonov koeficijent
Joule-Thomson coefficient (μ) is a parameter which describes the temperature change when a gas expands adiabatically through a nozzle from a high pressure to a low pressure region. It is defined by
where H is enthalpy.
Citing this page:
Generalic, Eni. "Vodeni plin." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table