conditional electrode potential → uvjetni elektrodni potencijal
Conditional or formal electrode potential (E°’) is equal to electrode potential (E) when overall concentrations of oxidised and reduced form in all its forms in a solution are equal to one. Conditional electrode potential includes all effects made by reactions that do not take part in the electron exchange, but lead to change of ion power, changes of pH, hydrolysis, complexing, precipitating, etc.
At 298 K (25 °C) and by converting natural (Napierian) logarithms into decimal (common, or Briggian) logarithms, Nernst’s equation for electrode potential can be written as follows:
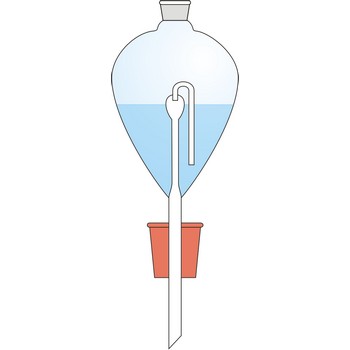
Contat-Gockel’s valve → Contat-Gockelov ventil
Contat-Göckel’s valve is used for maintenance of inert atmosphere in a flask. The valve is filled with a saturated solution of sodium bicarbonate (NaHCO3) so that the end of the tube is covered. Solution inside the valve keeps the flask contents away from the oxygen influence from air. If low pressure is created inside the flask (when the flask is cooled), the solution will penetrate inside it from funnel and in a reaction with acid CO2 is generated which fills up the flask.
Solution from the funnel will keep penetrating until CO2 pressure in the flask is equalised with the outer pressure.
cryogenic fluid → kriogenski fluid
Cryogenic fluids are used for accessing low temperatures, usually below -150 °C. Cryogenic temperatures are achieved by the rapid evaporation of volatile liquids. The most common laboratory cryogenic fluids are liquid nitrogen (-196 °C). Nitrogen gas is colorless and odorless. The cloud formed when pouring liquid nitrogen is condensed water vapour from the air, not nitrogen gas.
Dalton’s law → Daltonov zakon
Dalton’s law of partial pressure says that the total pressure eof gaseous mixture is equal to the sum of all gases partial pressures which make that mixture on the condition that they do not interact.
For example, if dry oxygen gas at 900 hPa is saturated with water vapor at 56 hPa, the pressure of the wet gas is 956 hPa.
dialysis → dijaliza
Dialysis is a method by which large molecules (such as starch or protein) and small molecules (such as glucose or amino acids) may be separated in a solution by selective diffusion through a semipermeable membrane. Through this kind of membrane dissolved particles pass and colloid dimension particles fall behind. For example, if a mixed solution of starch and glucose is placed in a closed container made of a semipermeable substance (such as cellophane), which is then immersed in a beaker of water, the smaller glucose molecules will pass trough the membrane into the water, while the starch molecules remain behind.
Grignard reagent → Grignardov reagens
Grignard reagents are organomagnesium halides, RMgX, having a carbon- magnesium bond (or their equilibrium mixtures in solution with R2Mg + MgX2).
heat of crystallization → toplina kristalizacije
Heat of crystallization or enthalpy of crystallization is the heat evolved or absorbed when one mole of given substance crystallises from a saturated solution of the same substance.
immersion plating → galvaniziranje uranjanjem
Immersion plating involves depositing a metallic coating on a metal immersed in a liquid solution, without the aid of an external electric current. Also called dip plating.
isomorphism → izomorfija
Isomorphism is the existence of two or more substances that have the same crystal structure, so that they form solid solutions.
Citing this page:
Generalic, Eni. "Vodena otopina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table