Brinell hardness → Brinellova tvrdoća
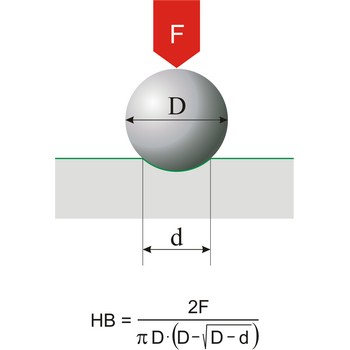
Brinell hardness is a scale for measuring the hardness of metals introduced around 1900 by Swedish metallurgist Johan Brinell (1849-1925). A small chromium steel ball is pressed into the surface of the metal by a load of known weight. The loading force is in the range of 300 N to 30 000 N. The ratio of the mass of the load in kilograms to the area of the depression formed in square millimetres is the Brinell Hardness Number.
Buchner flask → Buchnerova tikvica
Büchner flask (also known as a vacuum flask, filter flask, side-arm flask or Kitasato flask) is a thick-walled Erlenmeyer flask with a side arm to which a vacuum can be applied.
Buchner funnel → Buchnerov lijevak
Büchner funnel is one device used for pressure assisted filtration. Buchner funnel is a cylindrical porcelain filtering funnel (glass and plastic funnels are also available) that has a perforated plate on which the flat filter paper is placed. A vacuum in the flask underneath the filter allows atmospheric pressure on the sample to force the liquid through the filter paper. It is named after the German chemist Ernst Wilhelm Büchner (1850-1925) who designed this funnel in 1885.
Bunsen burner → Bunsenov plamenik
Bunsen burner is a standard source of heat in the laboratory. German chemist Roberts Bunsen (1811-1899) improved the burner's design, which had been invented by Faraday, to aid his endeavors in spectroscopy. The Bunsen burner has a vertical metal tube through which a fine jet of fuel gas is directed. Air is drawn in through airholes near the base of the tube and the mixture is ignited and burns at the tube’s upper opening. The flow of this air is controlled by an adjustable collar on the side of the metal tube. When the whole is closed a yellow safety flame is displayed. Where as when the whole is open it displays a power dull blue flame with a faint blue outer flame with a vibrant blue core used u for combustion and hearting. The flame can reach temperatures of 1 500 °C.
Bunsen’s cell → Bunsenov članak
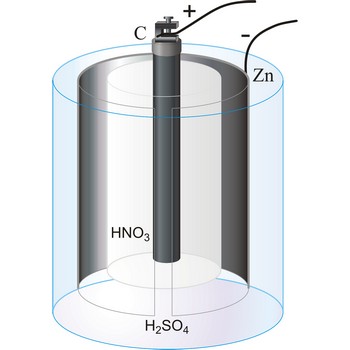
Bunsen’s cell is a primary cell devised by Robert W. Bunsen consisting of a zinc cathode immersed in dilute sulphuric acid and carbon anode immersed in concentrated nitric acid. The electrolytes are separated by a porous pot. The cell gives an e.m.f. of about 1.9 V.
burette → bireta
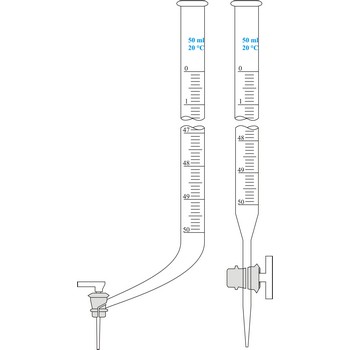
Burette is a graded glass pipe which on its lower side has a glass faucet by which it can drop a precise quantity of liquid. Inner diameter of a burette must be equal in its whole length, because the accuracy of volume measurement depends upon that. Burettes are primarily used in volumetric analysis for titration with standard solution reagent. Most often Schellbach’s burette is used, graded on 50 mL with division of scale on 0.1 mL. Every burette is calibrated on discharge. For serial determining automatic burettes are used.
calendering → kalandriranje
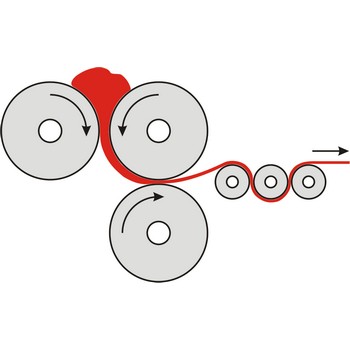
Calendering is the process of forming materials to make a film/sheet by passing them through a series of hot rollers.
calomel electrode → kalomel elektroda
Calomel electrode is a type of half cell in which the electrode is mercury coated with calomel (Hg2Cl2) and the electrolyte is a solution of potassium chloride and saturated calomel. In the calomel half cell the overall reaction is
Table: Dependence of potential of calomel electrode upon temperature and concentration of KCl according to standard hydrogen electrode
| Potential vs. SHE / V | |||
|---|---|---|---|
| t / °C | 0.1 mol dm-3 | 3.5 mol dm-3 | sat. solution |
| 15 | 0.3362 | 0.254 | 0.2511 |
| 20 | 0.3359 | 0.252 | 0.2479 |
| 25 | 0.3356 | 0.250 | 0.2444 |
| 30 | 0.3351 | 0.248 | 0.2411 |
| 35 | 0.3344 | 0.246 | 0.2376 |
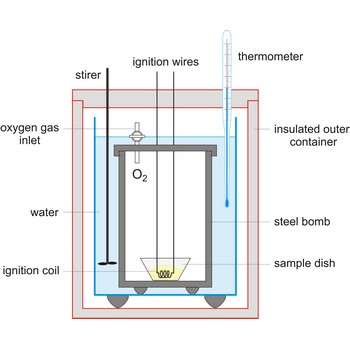
calorimeter → kalorimetar
Calorimeter is an instrument used to measure the energy absorbed or released in a chemical reaction. It also used in determining specific heat.
capacitor → kondenzator
Capacitor is a device that stores electric charges. The symbol for a capacitor in electric circuit schemes is —| |—.
Citing this page:
Generalic, Eni. "Visoko fruktozni kukuruzni sirup." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table