concentrated solution → koncentrirana otopina
Concentrated solution is a solution that contains a large amount of solute relative to the amount that could dissolve.
acetal → acetal
Acetals are organic compounds having the structure R2C(OR’)2 (R’ ≠ H). They are organic compounds formed by addition of alcohol molecules to aldehyde or ketone molecules. Originally, the term was confined to derivatives of aldehydes (one R = H), but it now applies equally to derivatives of ketones (neither R = H ). Mixed acetals have different R’ groups. The formation of acetals is reversible; acetals can be hydrolysed back to aldehydes (ketone) in acidic solutions.
Acetal, 1,1-diethoxyethane (CH3CH(OC2H5)2), is an organic compound, pleasant smelling, formed by addition of ethyl alcohol to ethanal (acetaldehyde). It is used as a solvent and in synthetic organic chemistry.
Acheson process → Achesonov proces
Acheson process is an industrial process to synthesize graphite and silicon carbide (carborundum), named after its inventor the American chemist Edward Goodrich Acheson (1856-1931). In this process, a solid-state reaction between pure silica sand (SiO2) and petroleum coke (C) at very high temperature (more than 2500 °C) leads to the formation of silicon carbide under the general reaction:
While studying the effects of high temperature on carborundum, Acheson had found that silicon vaporizes at about 4150 °C, leaving behind graphitic carbon.
acid-base titration → kiselo-bazna titracija
Acid-base titration is an analytical technique in volumetric analysis, where an acid of known concentration is used to neutralise a known volume of a base, and the observed volume of the acid required is used to determine the unknown concentration of the base. An acid-base indicator is used to determine the end-point of the titration.
cracking cotton → praskavi pamuk
Cracking cotton is nitrocellulose with high contents of nitrogen (around 13 %). It is used for the production of smokeless gunpowder.
fire-damp → plin praskavac
Fire-damp is a mixture of two volume parts of hydrogen and one volume part of oxygen which, if set on fire, strongly explodes, the flame giving of a very high temperature (2 000 °C).
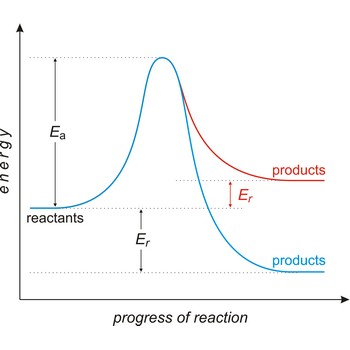
activation energy → energija aktivacije
Activation energy (Ea) is the energy that must be added to a system in order for a process to occur, even though the process may already be thermodynamically possible. In chemical kinetics, the activation energy is the height of the potential barrier separating the products and reactants. It determines the temperature dependence on the reaction rate.
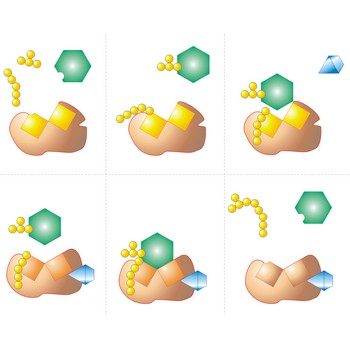
active site → aktivno mjesto
Active site is a pocket or crevice on an enzyme molecule that fits reactant molecules like a hand in a glove. The active site lowers the activation energy for reaction
Citing this page:
Generalic, Eni. "Visoka peÃâ¡." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table