Bunsen, Robert Wilhem → Bunsen, Robert Wilhem
Robert Wilhem Bunsen (1811-1899) is a German chemist who held professorships at Kassel, Marburg and Heidelberg. His early researches on organometallic compound of arsenic cost him an eye in an explosion. Bunsen's most important work was in developing several techniques used in separating, identifying, and measuring various chemical substances. He also improvement chemical battery for use in isolating quantities of pure metals - Bunsen battery.
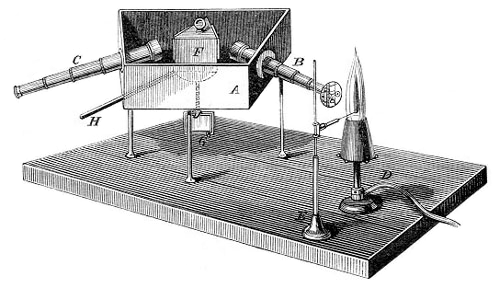
The essential piece of laboratory equipment that has immortalized the name of Bunsen was not invented by him. Bunsen improved the burner's design, which had been invented by Faraday, to aid his endeavors in spectroscopy. Use of the Bunsen burner in conjunction with a glass prism led to the development of the spectroscope in collaboration with the German physicist Gustav Kirchoff and to the spectroscopic discovery of the elements rubidium (1860) and cesium (1861).
geochemistry → geokemija
Geochemistry is the scientific study of the chemical composition of the Earth. It includes the study of the abundance of the Earth’s elements and their isotopes and the distribution of the elements in environments of the Earth (lithosphere, atmosphere, biosphere, and hydrosphere).
heat of formation → toplina nastajanja
Heat of formation or enthalpy of is formation the heat evolved or absorbed when one mole of a compound is formed in its standard state from its constituent elements.
heterocyclic compounds → heterociklički spojevi
Heterocyclic compounds are cyclic compounds having as ring members atoms of at least two different elements, e.g., quinoline, 1,2-thiazole.
impure substance → nečista tvar
Impure substance is every substance that contains traces of other elements or compounds in itself.
cerium → cerij
Cerium was discovered by Martin Heinrich Klaproth (Germany) and by Jöns Jacob Berzelius (Sweden) in 1803 and Wilhelm von Hisinger (Germany) in 1814. Named after the asteroid Ceres this discovered two years before the element. It is malleable, ductile, iron-grey metal. Tarnishes in air; reacts easily with water. Dissolves in acids; ignites when heated. Metal ignites and burns readily. Strong reductant. Cerium is most abundant rare earth metal. Found in many minerals like monazite sand [Ce(PO4)]. Its oxides are used in the optics and glass-making industries. Its salts are used in the photography and textile industry. Used in high-intensity carbon lamps and as alloying agents in special metals.
chemical compound formula → formula kemijskog spoja
Chemical elements are represented by their symbols, and chemical compounds are represented by a group of symbols of those elements from which the compound is composed. That group of symbols, which shows which atoms and in which number relation they are present in certain compound is called a chemical compound formula.
In a formula chemical symbols show which element is present in a certain compound, and its index shows how much of that element there is in a certain compound. From sulphuric acid formula H2SO4 we can see that one molecule of sulphuric acid consists of two atoms of hydrogen, one atom of sulphur and four atoms of oxygen.
control rod → kontrolna šipka
Control rods are rods in a nuclear reactor composed of substances that absorb neutrons (cadmium, hafnium, boron, etc.). These rods regulate the power level of the reactor.
Citing this page:
Generalic, Eni. "Vi element." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table

