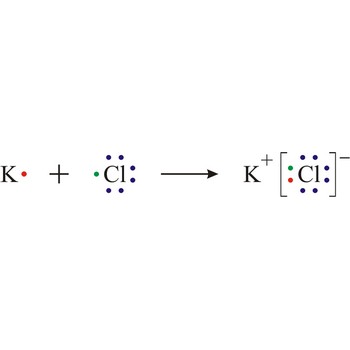insulator → izolator
Insulator is a material in which the highest occupied energy band (valence band) is completely filled with electrons, while the next higher band (conduction band) is empty. Solids with an energy gap of 5 eV or more are generally considered as insulators at room temperature. Their conductivity is less than 10-6 S/m and increases with temperature.
lanthanides contraction → kontrakcija lantanoida
Lanthanides contraction is a reduction of metal and ion diameters from lanthanum to lutetium and it is caused by a core charge growth inside the same shell. Elements which in the periodic system of elements come after lanthanides have, because of lanthanides contraction, smaller diameter than they should have according to their position in the periodic system of elements.
electron configuration → elektronska konfiguracija
The electron configuration shows how many electrons there are in an atom or ion and their distribution along orbitals (see Table of electronic configuration of elements). Structure and all regularity in the periodic system depend upon electronic configuration of atoms of elements. Characteristics of elements mainly depend on electronic configuration of the outer shell. Refilling of the new electronic shell atoms of elements of similar electronic configuration emerge as well as in the previous shell, which adds up to periodicities of characteristics of elements.
groups in periodic system of elements → skupine periodnog sustava
Periodic system of elements is divided into 18 groups of chemical elements. Elements belonging to the same group have a same number of valence electrons and similar chemical properties. Elements of main groups are in 1., 2., and in groups 13. to 18. Different groups of elements can be named according to the first element in the group (elements of boron group, elements of carbon group), or they have some special names (noble gases, halogenic elements, halyde elements, earthalkali and alkali metals).
ionisation → ionizacija
Ionisation is the process of producing ions. Certain molecules ionise in a solution; for example, acids ionise when dissolved in water.
Electron transfer also causes ionisation in certain reactions, for example sodium and chlorine react by transfer of a valence electron from the sodium atom to the chlorine atom to form the ions that constitute a sodium chloride crystal.
Lewis structure → Lewisova struktura
Lewis structure is the representation of the electron arrangement in atoms, ions, or molecules by showing the valence electrons as dots placed around the symbols for the elements.
metallic bond → metalna veza
Metallic bond is a electrostatic attraction binding the positive ions of a solid metal together by means of a "sea" of delocalised valence electrons
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
orbital → orbitala
Orbital is the area in space about an atom or molecule in which the probability of finding an electron is greatest.
The possible atomic orbitals correspond to subshells of the atom. Thus there is one s-orbital for each shell (orbital quantum number l = 0). There are three p-orbitals (corresponding to the three values of l) and five d-orbitals. The shapes of orbitals depend on the value of l.
Citing this page:
Generalic, Eni. "Valentna ljuska." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table