electrode potential → elektrodni potencijal
Electrode potential is defined as the potential of a cell consisting of the electrode in question acting as a cathode and the standard hydrogen electrode acting as an anode. Reduction always takes place at the cathode, and oxidation at the anode. According to the IUPAC convention, the term electrode potential is reserved exclusively to describe half-reactions written as reductions. The sign of the half-cell in question determines the sign of an electrode potential when it is coupled to a standard hydrogen electrode.
Electrode potential is defined by measuring the potential relative to a standard hydrogen half cell
The convention is to designate the cell so that the oxidised form is written first. For example
The e.m.f. of this cell is
By convention, at p(H2) = 101325 Pa and a(H+) = 1.00, the potential of the standard hydrogen electrode is 0.000 V at all temperatures. As a consequence of this definition, any potential developed in a galvanic cell consisting of a standard hydrogen electrode and some other electrode is attributed entirely to the other electrode
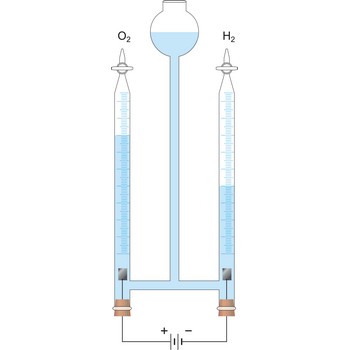
electrolysis → elektroliza
Electrolysis is the decomposition of a substance as a result of passing an electric current between two electrodes immersed in the sample.
electrolytes → elektroliti
Electrolytes are substances which, when melted or dissolved in water, conduct electric current. By melting or dissolving they are dissociated into electrically charged particles (ions) which are able to conduct electric current. By passing of electric current the transfer of matter occurs. Positively charged particles (cations) travel towards the negative pole (the cathode) and negatively charged particles (the anions) travel towards the positive pole (the anode). Liquid metals, in which the conduction is by free electrons, are not usually regarded as electrolytes. Solid conductors of ions, as in the sodium-sulphur cell, are also known as electrolytes. Depending upon how it conducts electric current, matter can be divided into strong electrolytes, weak electrolytes and nonconductors.
thermal pollution → toplinsko zagađenje
Thermal pollution is the increase in temperature of natural waters resulting from the discharge to these waters of hot effluents from industrial and power plants. The higher temperatures reduce the concentration of dissolved oxygen.
electronegativity → elektronegativnost
Electronegativity is a parameter originally introduced by L. Pauling which describes, on a relative basis, the power of an atom to attract electrons. For example, in hydrogen chloride, the chlorine atom is more electronegative than the hydrogen and the molecule is polar, with a negative charge on the chlorine atom.
There are various ways of assigning values for the electronegativity of an element. Pauling electronegativities are based on bond dissociation energies using a scale in which fluorine, the most electronegative element, has the value 4 and francium, the lowest electronegative element, has the value 0.7.
ether → eter
Ethers are organic compounds with a formula R-O-R, where R is not equal to H. They may be derived from alcohols by elimination of water, but the major method is catalytic hydration of olefins. They are volatile highly flammable compounds; when containing peroxides they can detonate on heating. The term ether is often used synonymously with diethyl ether.
europium → europij
Europium was discovered by Eugene Demarcay (France) in 1896. Named for the continent of Europe. It is soft, silvery-white metal. Extremely reactive with oxygen and water. Europium is obtained from monazite sand, which is a mixture of phosphates of calcium, thorium, cerium and most other rare earths. Used with yttrium oxide to make red phosphors for colour televisions.
Fahrenheit scale → Fahrenheitova skala
Fahrenheit scale is the temperature scale in which 212 degrees is the boiling point of water and 32 degrees is the freezing point of water. The scale was invented in 1714 by the German physicist G.D. Fahrenheit (1686-1736).
32 °F = 0 °C
212 °F = 100 °C
1 °F =(5/9) °C
T(°C) = (5/9)[T(°F) - 32]
T(°F) = (9/5)T(°C) + 32
Citing this page:
Generalic, Eni. "Tvrdoća vode." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table