quantum chemistry → kvantna kemija
Quantum chemistry is a theoretical branch of chemistry that concerns the application of quantum mechanics to chemical problems.
mass-energy equivalence → ekvivalencija mase i energije
In the special theory of relativity Einstein demonstrated that neither mass nor energy were conserved separately, but that they could be traded one for the other and only the total "mass-energy" was conserved. The relationship between the mass and the energy is contained in what is probably the most famous equation in science,
Where m is the mass of the object and c is the velocity of light. Cockcroft and Walton (1932) are routinely credited with the first experimental verification of mass-energy equivalence.
molecular shape → oblik molekule
Molecular shape is the three dimensional arrangement of atoms in space around a central atom. The molecular formula of a substance does not give an indication of its shape. For example, CO2 is a linear molecule, but SO2 is angular.
The three-dimensional shapes of many small molecules can be predicted by applying the valence shell electron pair repulsion theory (VSEPR). When atoms combine to form molecules, pairs of valence electrons arrange themselves as far from each other as possible. Another way to characterize molecular shape is in terms of hybrid orbitals.
Onsager relations → Onsagerove relacije
Onsager relations are an important set of equations in the thermodynamics of irreversible processes. They express the symmetry between the transport coefficients describing reciprocal processes in systems with a linear dependence of flux (Ji) on driving forces (Xj).
In Onsager’s theory the coupling coefficients are equal, Lij = Lji. This is known as reciprocal relations. The theory was developed by the Norwegian chemist Lars Onsager (1903-1976) in 1931.
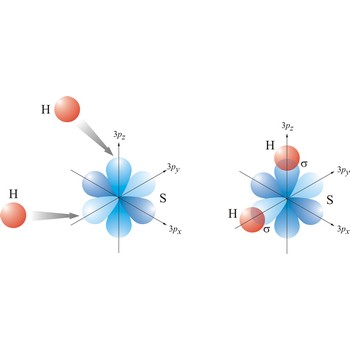
sigma bond → sigma veza
Most single bonds are sigma bonds (σ-bond). In the valence bond theory, a sigma bond is a valence bond that is symmetrical around the imaginary line between the bonded atoms.
valence bond → valentna veza
In the valence bond theory, a valence bond is a chemical bond formed by overlap of half-filled atomic orbitals on two different atoms.
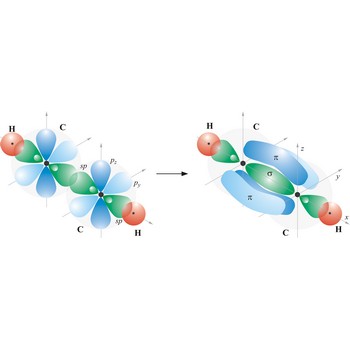
triple bond → trostruka veza
Triple bond. (≡) is a covalent bond that involves 3 bonding pairs. In the valence bond theory, one of the bonds in a triple bond is a sigma bond and the other two are pi bonds. For example, the central bond in acetylene is a triple bond: H-C≡C-H.
Citing this page:
Generalic, Eni. "Teorija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table