hydrosphere → hidrosfera
Hydrosphere (from the Greek for water sphere) is a discontinuous layer of water on, under, and over the Earth's surface. It includes all liquid and frozen surface waters, groundwater held in soil and rock, and atmospheric water vapour. Water continuously circulates between these reservoirs in what is called the hydrologic cycle, which is driven by energy from the Sun.
| Reservoir | V / 106 km3 | w / % |
|---|---|---|
| oceans | 1 370.0 | 97.25 |
| ice caps and glaciers | 29.0 | 2.05 |
| groundwater | 9.5 | 0.68 |
| lakes, rivers | 0.127 | 0.01 |
| soil moisture | 0.065 | 0.005 |
| atmosphere (as liquid equivalent of water vapour) | 0.013 | 0.001 |
| biosphere | 0.0006 | 0.00004 |
| TOTAL | 1 408.7 | 100 |
Joule-Thomson’s effect → Joule-Thomsonov efekt
Temperature of ideal gas will not be changed when it is repressed to a lower pressure, but when real gases are repressed to a lower pressure, a lower or higher temperature change appears under high pressures. The temperature change which appears at real gas expansion in a system into which energy is not brought is called Joule-Thomson’s effect. It was determined that when air is repressed by 1 bar, its temperature drops by 0.25 °C. That minute effect is completely irrelevant for most technical processes, but is also used in gas liquefying procedure.
Kirchoff, Gustav → Kirchoff, Gustav
Gustav Kirchoff (1824-1887) was a German physicist who, with the chemist Robert Bunsen (1811-1899), laid the foundations of spectral analysis. He realized that the Fraunhofer lines in the Sun's spectrum were due to light from the photosphere being absorbed at those specific wavelengths by elements in the solar atmosphere. He also found that incandescent solids, liquids, and compressed gases emit a continuous spectrum. Use of the Bunsen burner in conjunction with a glass prism led to the development of the spectroscope in collaboration with the Bunsen and to the spectroscopic discovery of the elements rubidium (1860) and cesium (1861).
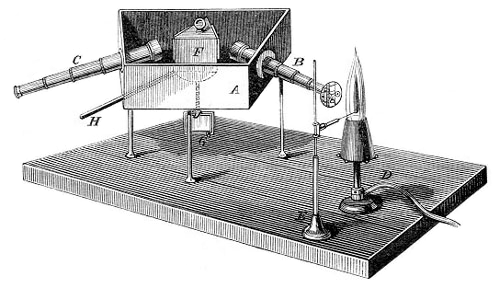
Knudsen’s pipette → Knudsenova pipeta
Kudsen's automatic pipette, developed by the Danish physicist Martin Knudsen (1871-1949), allows quick and accurate transfer of a constant volume of liquid (sea water), usually around 15 mL. On the top of pipette is a double sided C vent that can establish flow between the body of the pipette and one of the branches (A or B), or isolate the body of the pipette from both of the branches. Sucking through the B branch the pipette is filled with liquid, it is closed with a twist of the C valve and the liquid is released by rotating the valve towards the A branch (so atmospheric air can enter the pipette). Emptying the pipette takes around 30 seconds. Before it's first use, the pipette must be calibrated with distilled water.
Knudsen burette → Knudsenova bireta
Knudsen's automatic bulb-burette, developed by the Danish physicist Martin Knudsen (1871-1949), is designed in a way that even routine field analysis in a boat laboratory would provide highly accurate measurements. The burette is filled with a mixture of silver nitrate from reservoir R, located above the burette, by opening the A valve. When the solution crosses the three-way C valve the A valve is closed preventing further solution flow in to the burette. Any extra solution is caught in the W bowl. Turn the C valve, which marks the zero on the scale, in order to allow atmospheric air to enter the burette. Since most open-ocean samples lie in a relatively small chlorinity range, the burette is designed so that much of its capacity is in the bulb (B). This allows the titration to be quick (by quickly releasing contents from the B area) and reduces the error that occurs from the slow drainage along the inner wall of the burette.
Each millimeter is divided in to twenty parts (double millimeter division of the Knudsen burette) which allows for highly accurate measurements (the scale is read up to a precision of 0.005 mL). From 0 to 16 the burette isn't divided, that usually starts from 16 and goes until 20.5 or 21.5. A single double millimeter on a Knudsen burette scale corresponds to one permille of chloride in the seawater sample. This burette can be used for titration of water from all of the oceans and seas, with the exemptions being areas with very low salinity (e.g. the Baltic Sea) and river estuaries which require the use of normal burettes.
practical salinity → praktični salinitet
Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution). When K15 = 1, the Practical Salinity P S is by definition 35. The conductivity of that reference solution is C(35,1568,0) = 42.914 mS/cm = 4.2914 S/m (Siemens per meter). Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". When K15 is not unity, SP and K15 are related by the PSS-78 equation
At a temperature of t68 = 15 °C, Rt is simply K15 and Practical Salinity SP can be determined from the above equation. For temperatures other than t68 = 15 °C, Practical Salinity SP is given by the following function of Rt (k = 0.0162)
stratosphere → stratosfera
Stratosphere is the part of the earth’s atmosphere extending from the top of the troposphere (typically 10 km to 15 km above the surface) to about 50 km. It is characterised by an increase in temperature with increasing altitude.
thermosphere → termosfera
Thermosphere is the layer of the earth’s atmosphere extending from the top of the mesosphere (80 km - 90 km above the surface) to about 500 km. It is characterised by a rapid increase in temperature with increasing altitude up to about 200 km, followed by a levelling off in the 300 km - 500 km region.
salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
Citing this page:
Generalic, Eni. "Tehnička atmosfera." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table