bismuth → bizmut
Bismuth was discovered by Claude Geoffroy (France) in 1753. The origin of the name comes from the German words Weisse Masse meaning white mass; now spelled wismut and bisemutum. It is hard, brittle, steel-grey metal with a pink tint. Stable in oxygen and water. Dissolves in concentrated nitric acid. Bismuth can be found free in nature and in minerals like bismuthine (Bi2S3) and in bismuth ochre (Bi2O3) Main use is in pharmaceuticals and low melting point alloys used as fuses.
francium → francij
Francium was discovered by Marguerite Perey (France) in 1939. Named for France, the nation of its discovery. It is highly rare and unstable, radioactive metal. Chemical properties similar to cesium. Francium is formed by decay of actinium. Produced by bombarding radium or astatine with neutrons. Since its isotopes have such short half-lives there are no commercially significant compounds of francium.
holography → holografija
Holography is a technique for creating a three-dimensional image of an object by recording the interference pattern between a light beam diffracted from the object and a reference beam. The image can be reconstructed from this pattern by a suitable optical system.
blast furnace → visoka peć
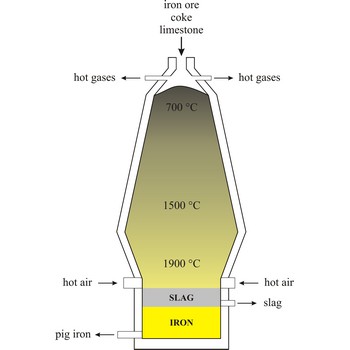
Blast furnace is a furnace for smelting of iron from iron oxide ores (hematite, Fe2O3 or magnetite, Fe3O4). Coke, limestone and iron ore are poured in the top, which would normally burn only on the surface. The hot air blast to the furnace burns the coke and maintains the very high temperatures that are needed to reduce the ore to iron. The reaction between air and the fuel generates carbon monoxide. This gas reduces the iron(III) oxide in the ore to iron.
Because the furnace temperature is in the region of 1500 °C, the metal is produced in a molten state and this runs down to the base of the furnace.
The production of iron in a blast furnace is a continuous process. The furnace is heated constantly and is re-charged with raw materials from the top while it is being tapped from the bottom. Iron making in the furnace usually continues for about ten years before the furnace linings have to be renewed.
boron → bor
Boron compounds have been known for thousands of years, but the element was not discovered until 1808 by Sir Humphry Davy (England) and independently by Joseph-Louis Gay-Lussac (France) and L. J. Thenard (France). The origin of the name comes from the Arabic word buraq and the Persian word burah meaning boraks. It is hard, brittle, lustrous black semimetal. Unreactive with oxygen, water, alkalis or acids. Combines with most metals to form borides. Boron is obtained from kernite, a kind of borax (Na2B4O7·10H2O). High purity boron is produced by electrolysis of molten potassium fluroborate and potassium chloride (KCl). Amorphous boron is used in pyrotechnic flares to provide a distinctive green color and in rockets as an igniter.
bromine → brom
Bromine was discovered by Antoine J. Balard (France) in 1826. The origin of the name comes from the Greek word bromos meaning stench. It is reddish-brown liquid with suffocating, irritating fumes. Gives off poisonous vapour. Causes severe burns. Oxidizer. Bromine occurs in compounds in sea water. It was once used in large quantities to make a compound that removed lead compound build up in engines burning leaded gasoline. Now it is primarily used in dyes, disinfectants and photographic chemicals.
Citing this page:
Generalic, Eni. "Svjetlosna godina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table