petroleum ether → petroleter
Petroleum ether is the petroleum fraction consisting of C5 and C6 hydrocarbons and boiling in the range 35 °C to 60 °C; commonly used as a laboratory solvent.
disaccharide → disaharid
Disaccharides are compounds in which two monosaccharides are joined by a glycosidic bond. A glycosidic bond to the anomeric carbon can be either α or β. For example, maltose, the disaccharide obtained by enzyme-catalyzed hydrolysis of starch, consists of two D-glucopyranose units joined by a 1,4’-α-glycoside bond. The "prime" superscript indicates that C-4 is not in the same ring as C-1. Unlike the other disaccharides, sucrose is not a reducing sugar and does not exhibit mutarotation because the glycosidic bond is between the anomeric carbon of glucose and the anomeric carbon of fructose.
primary alcohol → primarni alkohol
Primary alcohols are alcohols where the hydroxyl group is attached to a primary carbon atom. Thus, it has the general structure, RCH2OH, where R is a hydrogen atom or an alkyl group.
secondary alcohol → sekundarni alkohol
Secondary alcohol is one in which the hydroxyl group (-OH) is attached to a secondary carbon atom (i.e. a carbon atom which has one hydrogen atom attached to it).
dry cell → suhi članak
Dry cell or Leclanche cell is a primary cell having a zinc anode, a carbon (graphite) cathode surrounded by manganese dioxide, and a paste containing ammonium chloride as electrolyte. The electromotive force (emf) produced by a dry cell is 1.5 V. Dry cell is not reversible and therefore have a limited operating life. It is invented by the French engineer Georges Leclanché (1839.-1882.) in 1866.
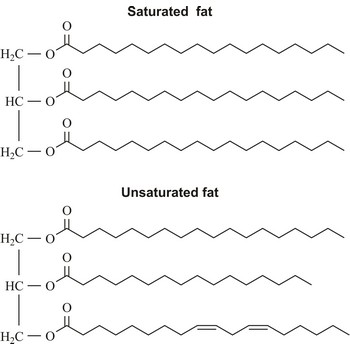
fat → mast
Fats are esters of glycerol and long chain carboxylic acids. Fats occur widely in plants and animals as a means of storing food energy, having twice the calorific value of carbohydrates. Fats derived from plants and fish generally have a greater proportion of unsaturated fatty acids than those from mammals. Fats may be either solid or liquid at room temperature, depending on their structure and composition. Unsaturated fats are liquid at room temperature.
Plant oils may be hardened by the addition of hydrogen atoms, converting double bonds to single bonds. This process is known as hydrogenation. Hydrogenated vegetable oils are often present in margarine and other processed foods.
Alkali hydrolysis of fat with sodium hydroxide it gives glycerol and soap (i.e. a mixture of the sodium salts of the fatty acids).
Citing this page:
Generalic, Eni. "Superkritični ugljikov dioksid." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table