redox potential → redoks potencijal
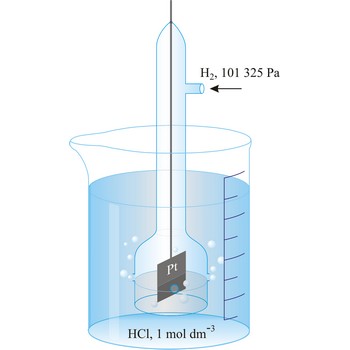
Redox potential is the potential of a reversible oxidation-reduction electrode measured with respect to a reference electrode, corrected to the hydrogen electrode, in a given electrolyte.
redox reaction → redoks-reakcija
Redox reaction is an oxidation-reduction reaction is a reaction in which one or more electrons are transferred. When an atom, ion, or molecule loses one or more electrons, it is oxidised. When an atom, ion, or molecule gains one or more electrons, it is reduced.
redox titration → redoks-titracija
Redox titration (oxidation-reduction titration) is a titration based on a redox reaction. For example, iron in water can be determined by converting dissolved iron to Fe2+ and titrating the solution with potassium permanganate (KMnO4), a powerful oxidising agent.
rhenium → renij
Rhenium was discovered by Walter Noddack, Ida Tacke and Otto Berg (Germany) in 1925. The origin of the name comes from the Latin word Rhenus meaning river Rhine. It is rare and costly, dense, silvery-white metal. Tarnishes in moist air. Resists corrosion and oxidation. Dissolves in nitric and sulfuric acids. Has a very high melting point. Rhenium is found in small amounts in gadolinite and molybdenite. Mixed with tungsten or platinum to make filaments for mass spectrographs. Its main value is as a trace alloying agent for hardening metal components that are subjected to continuous frictional forces.
superoxide → superoksid
Superoxides are binary compounds containing oxygen in the -½ oxidation state. Sodium superoxide (NaO2) can be prepared with high oxygen pressures, whereas the superoxides of rubidium, potassium, and cesium can be prepared directly by combustion in air. These compounds are yellow to orange paramagnetic solids. Superoxide ion, O2-, has an unpaired electron, is not particularly stable, and spontaneously decomposes into peroxide over time.
They are strong oxidising agents that vigorously hydrolyze (react with water) to produce superoxide and oxygen gas.
technetium → tehnecij
Technetium was discovered by Carlo Perrier and Emilio Segre (Italy) in 1937. The origin of the name comes from the Greek word technikos meaning artificial. It is silvery-grey metal. Resists oxidation but tarnishes in moist air and burns in high oxygen environment. First synthetically produced element. Radioactive. Technetium is made first by bombarding molybdenum with deuterons (heavy hydrogen) in a cyclotron. Added to iron in quantities as low as 55 part-per-million transforms the iron into a corrosion-resistant alloy.
Winkler’s method → Winklerova metoda
Winkler’s method was once a common method used to determine the dissolved oxygen concentration by titration. Now rarely used due to the accuracy and low price of oxygen meters.
The water sample is first treated with excess manganese(II) sulfate solution and then with an alkaline solution of potassium iodide. The Mn(OH)2 initially formed reacts with the dissolved oxygen. The amount of MnO(OH)2 formed is determined by reaction with iodide ion in acidic solution. The iodine formed may be titrated against standard thiosulfate solution, using starch as an indicator.
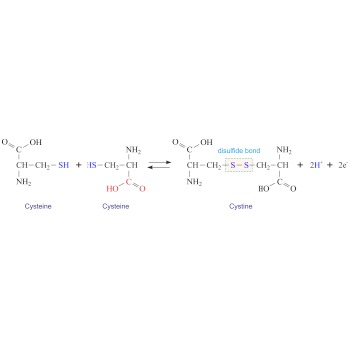
Cystine → Cistin
Cystine (C6H12N2O4S2) is a dimer of cysteine. It is formed by the oxidation of the thiol groups (-SH) of two cysteines generating a disulphide bridge (-S-S-). Cystine is a white crystalline solid that is slightly soluble in water. Cystine is particularly abundant in skeletal and connective tissues and in hair, horn, and wool.
Citing this page:
Generalic, Eni. "Stupanj oksidacije." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table