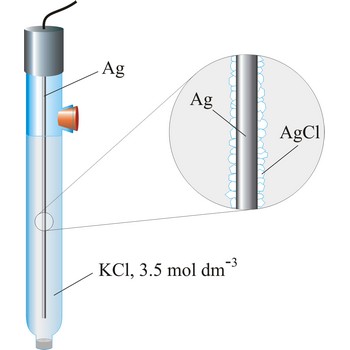
silver/silver-chloride electrode → srebro/srebrov klorid elektroda
Silver/silver-chloride electrode is by far the most common reference type used today because it is simple, inexpensive, very stable and non-toxic. It is mainly used with saturated potassium chloride electrolyte, but can be used with lower concentrations such as 3.5 mol dm-3 or 1 mol dm-3 potassium chloride. Silver/silver-chloride electrode is a referent electrode based on the following halfreaction
| Potential vs. SHE / V | ||
|---|---|---|
| t / °C | 3.5 mol dm-3 | sat. solution |
| 15 | 0.212 | 0.209 |
| 20 | 0.208 | 0.204 |
| 25 | 0.205 | 0.199 |
| 30 | 0.201 | 0.194 |
| 35 | 0.197 | 0.189 |
supercritical fluid → superkritični fluid
Supercritical fluid is any substance above its critical temperature and critical pressure (see phase diagram). It shows unique properties that are different from those of either gases or liquids under standard conditions. A supercritical fluid has both the gaseous property of being able to penetrate anything, and the liquid property of being able to dissolve materials into their components. Solublity increases with increasing density (i.e. with increasing pressure). An example of this is naphthalene which is practically insoluble in low pressure carbon dioxide. At 100 bar the solubility is 10 g/L and at 200 bar it is 50 g/L. Rapid expansion of supercritical solutions leads to precipitation of a finely divided solid.
thermometer → termometar
Thermometers are devices for measuring temperature. Linear and volume thermal expansion are macroscopic properties of matter, which can be easily measured, relative to measurements of microscopic properties, on the basis of which, temperature is defined. Thermometers based on thermal expansion are secondary instruments that is, they have to be calibrated in comparison to a standard thermometer. In a thermometer with liquid, mercury or alcohol is placed in a small glass container. If temperature increases, the liquid undergoes volume expansion and rises in a capillary. The level of the raised liquid is the measure of temperature. Mercury thermometers measure temperatures in the temperature range between -39 °C and 300 °C. Alcohol thermometers measure lower temperatures. Bimetal thermometers have a spiral spring, which consists of two metals with different coefficients of linear expansion. When temperature changes, metals undergo different change in length and the consequence twisting of the spring is transferred to a pointer, the deflection of which is the measure of temperature.
titar → titar
Titar (T) is a mass of titrated matter which is equivalent to 1 cm3 of solution. It is shown as T = 2.356 mg HCl / 1.0 cm3 NaOH, 0.1000 moldm-3, and it is usually shown in a table form. If the concentration of used standard solution (c) differs from one outlined in the table data (c0), the factor of correction (f) is induced
Titar is usually used in industrial operational laboratories where from titar tables mass or percentage of the ingredient in question is directly read.
van’t Hoff equation → van Hoffova jednadžba
Van’t Hoff equation is the equation expressing the temperature dependence on the equilibrium constant K of a chemical reaction:
where ΔrH° is the standard enthalpy of reaction, R the molar gas constant, and T the temperature.
volumetric flask → odmjerna tikvica
Volumetric flasks are bottles made of glass, in a pear like in shape with long thin necks and flat bottoms. All come with a ground glass stopper for a tight seal. Volume marking is cut in glass with fluoride acid around the neck, so that parallax should be avoided (flask is put in front of the eyes so that one can see only a straight horizontal line). A volumetric flask is calibrated to contain (TC or In) the indicated volume of water at 20 °C when the bottom of the meniscus is adjusted to just rest on the center of the line marked on the neck of the flask. They are used for preparing the exactly known volume of sample solution and standard solutions of reagents. On each flask with volume designation a temperature on which the flask has been calibrated is designated.
Winkler’s method → Winklerova metoda
Winkler’s method was once a common method used to determine the dissolved oxygen concentration by titration. Now rarely used due to the accuracy and low price of oxygen meters.
The water sample is first treated with excess manganese(II) sulfate solution and then with an alkaline solution of potassium iodide. The Mn(OH)2 initially formed reacts with the dissolved oxygen. The amount of MnO(OH)2 formed is determined by reaction with iodide ion in acidic solution. The iodine formed may be titrated against standard thiosulfate solution, using starch as an indicator.
Citing this page:
Generalic, Eni. "Standard." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table