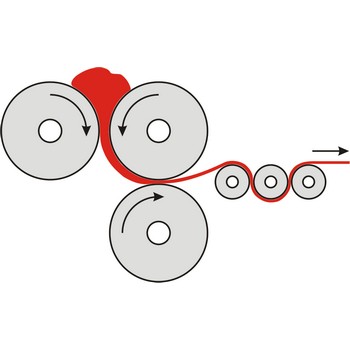
calendering → kalandriranje
Calendering is the process of forming materials to make a film/sheet by passing them through a series of hot rollers.
carbon → ugljik
Carbon has been known since ancient times. The origin of the name comes from the Latin word carbo meaning charcoal. Graphite form of carbon is a black, odourless, slippery solid. Graphite sublimes at 3825 °C. Diamond form is a clear or colored; an extremely hard solid. C60 is Buckminsterfullerine. Carbon black burns readily with oxidants. Carbon is made by burning organic compounds with insufficient oxygen. There are close to ten million known carbon compounds, many thousands of which are vital to organic and life processes. Radiocarbon dating uses the carbon-14 isotope to date old objects.
catalyst → katalizator
Catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. Catalysts that have the same phase as the reactants are homogenous catalysts (e.g. enzymes in biochemical reactions). Those that have a different phase are heterogeneous catalyst (e.g. metals or oxides used in gas reactions).
The catalyst provides an alternative pathway by which the reaction can proceed, in which the activation energy is lower. In thus increases the rate at which the reaction comes to an equilibrium, although it does not alter the position of the equilibrium.
computational chemistry → kompjutacijska kemija
Computational chemistry is a branch of chemistry concerned with the prediction or simulation of chemical properties, structures, or processes using numerical techniques.
concentration of ore → koncentriranje ruda
Concentration of ores is important industrial processes and is the first steps to the extraction of the metals. Normally, the ore is concentrated by separating it from the clay body in which it occurs either by gravity, sedimentation, or by a floatation process, before the extraction of the metal from the ore is started.
catalytic cracking → katalitčko krekiranje
Catalytic cracking is a petroleum refining process in which heavy-molecular weight hydrocarbons are broken up into light hydrocarbon molecules by the application of heat and pressure in the presence of a catalyst.
cathode → katoda
Cathode is a negative electrode of an electrolytic cell to which positively charged ions (cations) migrate when a current is passed as in electroplating baths.
In a primary or secondary cell (battery or accumulator) the cathode is the electrode that spontaneously becomes negative during discharge, and form which therefore electrons emerge.
In vacuum electronic devices electrons are emitted by the cathode and flow to the anode.
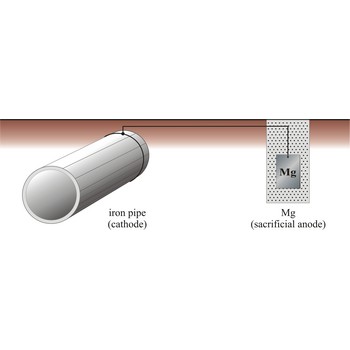
cathodic protection → katodna zaštita
Cathodic protection is a process in which a structural metal, such as iron, is protected from corrosion by connecting it to a metal that has a more negative reduction half-cell potential, which now corrodes instead of iron. There are two major variations of the cathodic method of corrosion protection. The first is called the impressed current method, and the other is called the sacrificial anode method.
cell potential → potencijal članka
Cell potential (E) is difference between anode and cathode potential. If the cell potential is positive, then the reaction is spontaneous.
conduction → kondukcija
This process occurs most significantly in solids. The atoms or molecules in a solid state do not leave their mean positions, but continue to vibrate about their mean positions. They transfer heat energy from one atom to another. This happens because of the coupling between them due to mutually attractive forces.
Citing this page:
Generalic, Eni. "Spontani proces." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table