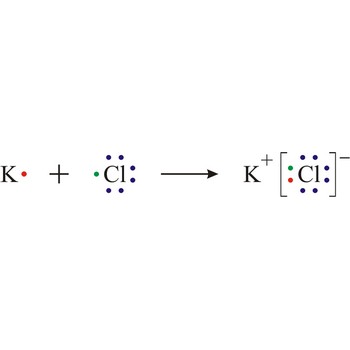hydration → hidratacija
Hydration is addition of water or the elements of water (i.e. H and OH) to a molecular entity. The term is also used in a more restricted sense for the process:
ionic bond → ionska veza
Ionic bond is a strong force of attraction holding atoms together in a molecule or crystal. Typically chemical bonds have energies of about 100 kJ mol-1. Ionic bond is a bond at which one of the participants, during the procedure of bonding, gives away its unpaired electrons to another atom so that both can achieve electron arrangement of the closest noble gas. In order to form an ionic bond one of the atoms must cross to the positively charged ion by losing certain number of electrons and the other atom must receive those electrons and cross to the negatively charged ion.
ionisation → ionizacija
Ionisation is the process of producing ions. Certain molecules ionise in a solution; for example, acids ionise when dissolved in water.
Electron transfer also causes ionisation in certain reactions, for example sodium and chlorine react by transfer of a valence electron from the sodium atom to the chlorine atom to form the ions that constitute a sodium chloride crystal.
Joule-Thomson’s effect → Joule-Thomsonov efekt
Temperature of ideal gas will not be changed when it is repressed to a lower pressure, but when real gases are repressed to a lower pressure, a lower or higher temperature change appears under high pressures. The temperature change which appears at real gas expansion in a system into which energy is not brought is called Joule-Thomson’s effect. It was determined that when air is repressed by 1 bar, its temperature drops by 0.25 °C. That minute effect is completely irrelevant for most technical processes, but is also used in gas liquefying procedure.
lime → živo vapno
Lime (or quicklime) is the common name for calcium oxide (CaO). It is manufactured from limestone, CaCO3, by heating it to a high temperature (about 1 000 °C). At this temperature carbon dioxide, CO2, is released from the limestone creating calcium oxide, CaO.
A further process involves adding water in a process known as hydrating, which produces hydrated, or slaked lime [Ca(OH)2].
Onsager relations → Onsagerove relacije
Onsager relations are an important set of equations in the thermodynamics of irreversible processes. They express the symmetry between the transport coefficients describing reciprocal processes in systems with a linear dependence of flux (Ji) on driving forces (Xj).
In Onsager’s theory the coupling coefficients are equal, Lij = Lji. This is known as reciprocal relations. The theory was developed by the Norwegian chemist Lars Onsager (1903-1976) in 1931.
paper chromatography → papirna kromatografija
Paper chromatography is one of the types of chromatography procedures which runs on a piece of specialized paper. It is a planar chromatography systems wherein a cellulose filter paper acts as a stationary phase on which separation of compounds occurs. The edge of the paper is immersed in a solvent, and the solvent moves up the paper by capillary action.
phosphorescence → fosforescencija
Phosphorescence is emission of light from a substance exposed to radiation and persisting as an afterglow after the exciting energy has been removed. Unlike fluorescence, in which the absorbed energy is spontaneously emitted about 10-8 second after excitation, phosphorescence requires additional excitation to produce radiation and may last from about mili second to days or years, depending on the circumstances.
pickling → dekapiranje
Pickling is a process to chemically remove scale or oxide from steel to obtain a clean surface. When applied to bars or coils prior to bright drawing, the steel is immersed in a bath of dilute sulphuric acid (w(H2SO4) = 10 %) heated to a temperature of around 80 °C. An inhibitor is added to prevent attack and pitting of the cleaned metal. After pickling, a washing process takes place followed by immersion in a lime-water bath to neutralise any remaining acid.
plasma → plazma
Plasma is a highly ionised gas in which the charge of the electrons is balanced by the charge of the positive ions, so that the system as a whole is electrically neutral. Plasmas are created by exposing gases at low pressure to an electric or electromagnetic field. In semiconductor processing, plasmas are used for etching and thin film deposition (the excited state of the gas makes it very reactive). In everyday life plasmas are used to give light in fluorescent light bulbs, neon lamps, and blue insect traps.
Citing this page:
Generalic, Eni. "Spontani proces." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table