hybrid orbital → hibridne orbitale
Hybrid orbital is an orbital created by mixing together atomic orbitals to form an equal number of new hybrid atomic orbitals. For example, a common hybridization is sp3 where s orbital combine with a three p orbitals to form four new orbitals. After hybridization, all hybrid orbitals have the same energy, lower than p orbitals, but higher than s orbitals.
sp hybrid orbital → sp hibridna orbitala
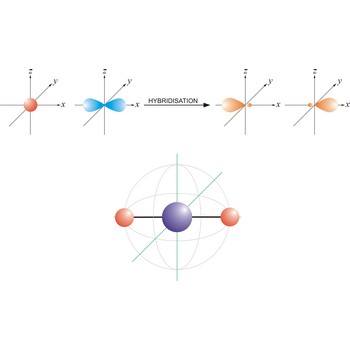
An sp hybrid orbital is an orbital formed by the linear combination of one s and one p orbital of comparable energy (such 2s and 2p orbitals) on a same atom. The two sp hybrid orbitals are aligned in a straight line in opposite direction (bond angles are 180°). The remaining two p orbitals are at right angles to one another and to the line formed by the two sp orbitals.
sp2 hybrid orbital → sp2 hibridna orbitala
An sp2 hybrid orbital is an orbital formed by the linear combination of one s and two p orbitals of comparable energy (such 2s and 2p orbitals) on a same atom. The three sp2 hybrid orbitals lie in a plane with angle of 120°. The remaining p orbital remains unchanged and is perpendicular to the plane of the three sp2 orbitals.
sp3 hybrid orbital → sp3 hibridna orbitala
An sp3 hybrid orbital is an orbital formed by the linear combination of one s and three p orbitals of comparable energy (such 2s and 2p orbitals) on a same atom. The four sp3 hybrid orbitals point toward the corners of a regular tetrahedron with the bond angle of 109.5°.
atomic orbital → atomska orbitala
Atomic orbital is a wave function that describes the behaviour of an electron in an atom.
orbital → orbitala
Orbital is the area in space about an atom or molecule in which the probability of finding an electron is greatest.
The possible atomic orbitals correspond to subshells of the atom. Thus there is one s-orbital for each shell (orbital quantum number l = 0). There are three p-orbitals (corresponding to the three values of l) and five d-orbitals. The shapes of orbitals depend on the value of l.
hybridization → hibridizacija
Hybridization is an internal linear combination of atomic orbitals, in which the wave functions of the atomic orbitals are added together to generate new hybrid wave functions. The new orbitals which are formed are hybrids of the originals and have properties (shape, size and energy) that are somewhere in between.
degenerate orbitals → degenerirane orbitale
Degenerate orbitals are orbitals with the same energy. This degeneracy can sometimes be "lifted" by external electric or magnetic fields.
valence bond theory → teorija valentne veze
Valence bond theory is a theory that explains the shapes of molecules in terms of overlaps between half-filled atomic orbitals, or half filled hybridised orbitals.
molecular shape → oblik molekule
Molecular shape is the three dimensional arrangement of atoms in space around a central atom. The molecular formula of a substance does not give an indication of its shape. For example, CO2 is a linear molecule, but SO2 is angular.
The three-dimensional shapes of many small molecules can be predicted by applying the valence shell electron pair repulsion theory (VSEPR). When atoms combine to form molecules, pairs of valence electrons arrange themselves as far from each other as possible. Another way to characterize molecular shape is in terms of hybrid orbitals.
Citing this page:
Generalic, Eni. "Sp3 hybrid orbital." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table